
J.of Supercritical Fluids 56 (2011) 41–47
Contents lists available at ScienceDirect
The Journal of Supercritical
Fluids
j o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /s u p f l
u
Reaction of d -glucose in water at high temperatures (410?C)and pressures (180MPa)for the production of dyes and nano-particles
Zhen Fang a ,?,Richard L.Smith Jr.b ,Janusz A.Kozinski c ,Tomoaki Minowa d ,Kunio Arai e
a
Biomass Group,1Xishuangbanna Tropical Botanical Garden,Chinese Academy of Sciences,88Xuefulu,Kunming,Yunnan province 650223,China
b Graduate School of Environmental Studies,Research Center of Supercritical Fluid Technology,Department of Chemical Engineering,Tohoku University,Aoba-ku Aramaki Aza Aoba-6-6-11,Sendai 980-8579,Japan c
Department of Earth and Space Science &Engineering,School of Engineering,York University,4700Keele Street,Toronto,Canada M3J 1P3d
Biomass Technology Research Laboratory,National Institute of Advanced Industrial Science and Technology,2-2-2Hiro Suehiro,Kure,Hiroshima 737-0197,Japan e
Graduate School of Environmental Studies,Tohoku University,Aoba-ku Aramaki Aza Aoba-04,Sendai 980-8579,Japan
a r t i c l e i n f o Article history:
Received 19May 2010
Received in revised form 25October 2010Accepted 13November 2010Keywords:Glucose
Hydrothermal
Diamond anvil cell Dyes Particles
Sub-and supercritical water Biomass
a b s t r a c t
An autoclave (120-mL)and an optical micro-reactor (50-nL)were used to study the hydrothermal decom-position of d -glucose at high temperatures and high pressures.During slow heating (0.18?C/s)to 350?C in the autoclave,water-soluble glucose (0.9M)began to decompose at 220?C and reacted completely at 280?C.The initial decomposition products were 5-(hydroxymethyl)furfural and levoglucosan,and these subsequently converted into oil and solid residue,and ?nally to solid particles at a 65wt%yield at 350?C.When the same heating rate and temperature were used on glucose solutions in the micro-reactor,yel-low and orange materials decomposed from glucose were produced.Numerous particles precipitated at 251?C,and at 350?C,all the glucose changed to an orange ?lm and solid particles,which were nanoparti-cles as con?rmed by SEM.However,when the glucose solution was rapidly heated to 410?C (9.5–17?C/s),yellow,brown and orange sugar-like materials were produced.A homogeneous phase with yellow color still remained at temperatures as high as 380?C,and few particles formed until 410?C.It can be concluded that micron-sized particles and colored solutions can be produced by slow heating,while rapid heating resulted in the formation of dye-like substances with glucose-like structures.The formation of colored solutions and particles may have technological implications in food or materials formation processes that use high temperature water with biomass feedstocks.
? 2010 Elsevier B.V. All rights reserved.
1.Introduction
There are many possible applications for using hydrothermal or high temperature water (HTW)as a solvent in thermal and chemical conversion processes [1,2].HTW behaves as a weakly polar solvent with both acidic and basic characters [3,4]and since its physic-ochemical properties can be widely varied with relatively small changes in temperature,HTW can replace many organic solvents.The properties of HTW and water in its supercritical state have been exploited for destruction of toxic organic wastes [5–7],syn-thesis of nanoparticles [8],production of organic chemicals [9,10],solubilization of biomass [11,12],hydrolysis of biomass-related compounds [12–14],gasi?cation of wood and biomass [15–19]and biomass liquefaction [17,18].
Glucose,which is a monosaccharide unit in many biopolymers,has been studied extensively in HTW for biofuels and chemicals
?Corresponding author.Tel.:+868715163360;fax:+868715160916.
E-mail addresses:zhenfang@https://www.doczj.com/doc/6a13542739.html, ,zhen.fang@mail.mcgill.ca (Z.Fang).1
https://www.doczj.com/doc/6a13542739.html,/.[16,17,19–38].Glucose can be converted to biofuels and a 100%gasi?cation rate has been demonstrated with activated carbon or KOH catalyst [23,24]or without catalyst at high temperatures (700?C)[32],but its liquefaction rate is generally low (ca.21%)with formic acid and cobalt catalyst at 300?C [33].Conversion rates of glucose to glycolaldehyde and erythrose are almost 50wt%by non-catalytic hydrothermal methods at 400?C and 30MPa [31,34].
Glucose can be reacted in water to obtain yields of 5-(hydroxymethyl)furfural (5-HMF)as high as 27%at 180?C in 3min reaction time [39],and when glucose is reacted in ionic liquid sol-vents with CrCl 2,5-HMF yields of 70%can be obtained at 100?C in 3h [40].Kabyemela et al.[35,36]give detailed reaction path-ways,mechanisms and kinetics of glucose reactions in HTW and supercritical water.The decomposition pathways are proposed as [18,35–38,41,42]:glucose isomerizes to fructose,or decomposes to erythrose,glycolaldehyde,dihydroxyacetone,glyceraldehyde,lev-oglucosan and pyruvaldehyde.The fructose formed decomposes to glyceraldehyde,dihydroxyacetone and erythrose.Then,the glycer-aldehyde and dihydroxyacetone dehydrate to pyruvaldehyde,and pyruvaldehyde,erythrose and levoglucosan decompose to acids,aldehydes and alcohols of 1–3carbons.Part of glucose degrades to
0896-8446/$–see front matter ? 2010 Elsevier B.V. All rights reserved.doi:10.1016/j.sup?u.2010.11.009
42Z.Fang et al./J.of Supercritical Fluids56 (2011) 41–47
furfurals and5-HMF,that condense to phenols and dehydrate to acids(e.g.,levulinic and formic acids).As reaction time increases, all of these compounds are highly reactive and readily polymer-ize to form water insoluble humic acids that are commonly called humins.Hydrothermal conversion of glucose to biofuels,chemicals and materials has been well studied.
Glucose has been used to study the formation of other chemical products that have technological interest.Non-catalytic reaction of glucose in water has been studied to produce colored compounds at 130?C for18h reaction time[43],and nanoparticles at170–180?C and0.7–0.8MPa for2h reaction time[44].However,few stud-ies have examined the possible types of solids formed under hydrothermal conditions.For example,it is not known for what conditions solids are formed and what type of properties they could https://www.doczj.com/doc/6a13542739.html,rmation on the conditions for homogeneous reaction and solid formation could provide a method for controlling glucose reaction products or possibly making new types of materials.In this work,non-catalytic decomposition of glucose was studied at temperatures up to410?C with the objective to produce?ne parti-cles and dye-like materials.Two types of reactors were used:(i)an autoclave(120mL)for the study of decomposition at long reaction times and slow heating rates,and(ii)a50-nL optical micro-reactor, hydrothermal diamond anvil cell for visual observation of the phase and color changes during the decomposition process.
2.Materials and methods
2.1.Materials
Glucose particles(>99.0%purity)used was from Fluka(Bio-Chemika).HPLC standard distilled water(Aldrich)had a maximum conductivity of5.5S m?1.Aqueous solutions of0.9-M glucose were made for the experiments.
2.2.Autoclave experiments
Aqueous glucose solutions(0.9-M,30mL)were reacted in a120-mL autoclave(18-mL dead space).An initial3-MPa N2 pressure was applied to the headspace to minimize water vapor-ization.After being sealed,the autoclave was heated to200–350?C (4.3–16.5MPa)at a heating rate of0.18?C/s and stirred by a mag-netic stirrer.After reaching the desired maximum temperature and holding time(0,30min,1h),the autoclave was cooled by turning off the electrical power.The gas volume was measured using a gas meter(Shinagawa corporation,W-NK-0.5B),and its composition was analyzed by gas chromatography(GC;Shimadzu,GC-12A with a TCD detector or GC-9A with an FID detector).The reaction mixture was separated into three phases:(i)aqueous phase,(ii)oil phase (acetone-soluble),and(iii)water-insoluble fraction(residue).The aqueous phase was analyzed for total organic carbon(TOC)with a Yanaco TOC-8L analyzer and for glucose with a Shimadzu UV-160A absorption spectrometer.After freeze-drying,sugars in the aqueous phase as trimethylsilyl derivatives were analyzed with a GC(Hewlett-Packard,5890A;CBP-10column).Organic elements (C,H)of the oil and residue were analyzed by an elemental ana-lyzer(AMCO NA-1500).Details of the experimental procedures have been described in a previous work[45].
2.3.Diamond anvil cell experiments
An optical micro-reactor,Bassett-type[46]hydrothermal dia-mond anvil cell(DAC)was used for visual observations of aqueous glucose solution during heating process.The reaction chamber, which consisted of a hole drilled in an Inconel gasket(50nL; 500-?m i.d.and250-?m thickness)loaded with glucose solution was sealed by compression with two opposing diamond
anvils,
10
20
30
40
50
60
70
200220240260280300320340350
Temperature (o C)
P
r
o
d
u
c
t
Y
i
e
l
d
s
(
c
a
r
b
o
n
,
%
)
0, 30m 1h
(holding time)
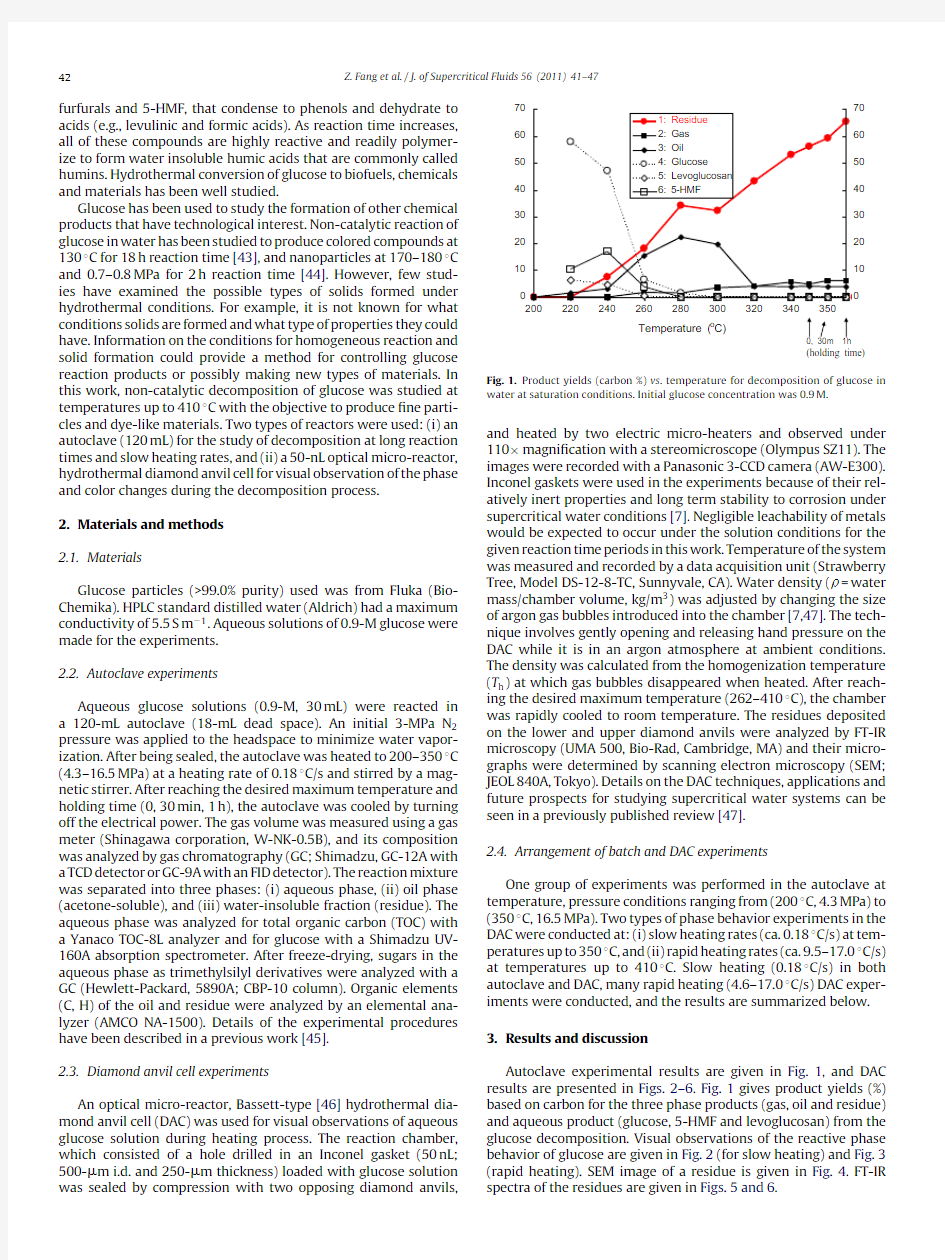
Fig.1.Product yields(carbon%)vs.temperature for decomposition of glucose in water at saturation conditions.Initial glucose concentration was0.9M.
and heated by two electric micro-heaters and observed under 110×magni?cation with a stereomicroscope(Olympus SZ11).The images were recorded with a Panasonic3-CCD camera(AW-E300). Inconel gaskets were used in the experiments because of their rel-atively inert properties and long term stability to corrosion under supercritical water conditions[7].Negligible leachability of metals would be expected to occur under the solution conditions for the given reaction time periods in this work.Temperature of the system was measured and recorded by a data acquisition unit(Strawberry Tree,Model DS-12-8-TC,Sunnyvale,CA).Water density( =water mass/chamber volume,kg/m3)was adjusted by changing the size of argon gas bubbles introduced into the chamber[7,47].The tech-nique involves gently opening and releasing hand pressure on the DAC while it is in an argon atmosphere at ambient conditions. The density was calculated from the homogenization temperature (T h)at which gas bubbles disappeared when heated.After reach-ing the desired maximum temperature(262–410?C),the chamber was rapidly cooled to room temperature.The residues deposited on the lower and upper diamond anvils were analyzed by FT-IR microscopy(UMA500,Bio-Rad,Cambridge,MA)and their micro-graphs were determined by scanning electron microscopy(SEM; JEOL840A,Tokyo).Details on the DAC techniques,applications and future prospects for studying supercritical water systems can be seen in a previously published review[47].
2.4.Arrangement of batch and DAC experiments
One group of experiments was performed in the autoclave at temperature,pressure conditions ranging from(200?C,4.3MPa)to (350?C,16.5MPa).Two types of phase behavior experiments in the DAC were conducted at:(i)slow heating rates(ca.0.18?C/s)at tem-peratures up to350?C,and(ii)rapid heating rates(ca.9.5–17.0?C/s) at temperatures up to410?C.Slow heating(0.18?C/s)in both autoclave and DAC,many rapid heating(4.6–17.0?C/s)DAC exper-iments were conducted,and the results are summarized below.
3.Results and discussion
Autoclave experimental results are given in Fig.1,and DAC results are presented in Figs.2–6.Fig.1gives product yields(%) based on carbon for the three phase products(gas,oil and residue) and aqueous product(glucose,5-HMF and levoglucosan)from the glucose decomposition.Visual observations of the reactive phase behavior of glucose are given in Fig.2(for slow heating)and Fig.3 (rapid heating).SEM image of a residue is given in Fig.4.FT-IR spectra of the residues are given in Figs.5and6.
Z.Fang et al./J.of Supercritical Fluids56 (2011) 41–47
43
Fig.2.Visual observation of{glucose+water}mixtures during slow heating up to350?C and136MPa(heating rate=0.18?C/s;water density=796kg/m3
).
Fig.3.Visual observation of{glucose+water}mixtures during rapid heating up to410?C and181MPa(heating rate=14.3?C/s;water density=768kg/m3
).
Fig.4.SEM image for the residue cooled from slow heating(0.18?C/s)glucose solu-tion to350?
C.
40080012001600200024002800320036004000
Wavenumber (cm-1)
A
b
s
o
r
b
a
n
c
e
Fig.5.IR spectra for residues a–e cooled from slow heating(0.18?C/s)glucose solu-tion up to350?C and30min holding time(curves denote,a–e:measurements after cooling from262,300,313,350?C and{350?C+30min})(subscripts;low:lower anvil;up:upper anvil).
44
Z.Fang et al./J.of Supercritical Fluids
56 (2011) 41–47400
800
1200
1600
2000
2400
2800320036004000
Wavenumber (cm -1
)
A b s o r b a n c e
Fig.6.IR spectra for residues a–e on lower anvils cooled from rapid heating (9.5–17.0?C/s)glucose solution up to 410?C (curves denote,a–c:measurements after cooling from 320,360and 410?C;d:medium heating at 4.6?C/s to 410?C;e:pyrolysis by rapid heating to 410?C).
Fig.7gives UV/vis absorbance spectra of initial glucose and decomposed glucose solutions.Fig.8shows reaction paths for the production of particles,gas and oil.The details of the experiments are discussed in the sections below.3.1.Autoclave experiments
Non-catalytic decomposition of glucose was studied with the autoclave by slow heating (heating rate of 0.18?C/s)from room temperature to the maximum temperature (200–350?C,up to 30-min heating time).Glucose started decomposing sharply at 220?C and completely disappeared at 280?C (Fig.1,curve 4).The initial decomposition products were 5-HMF and levoglucosan that subse-quently disappeared at 280?C (Fig.1,curves 5–6).A solid residue (Fig.1,curve 1)appeared at 240?C,and its yield gradually climbed to a value of 65%at 350?C and 1h reaction time.Oil yield (Fig.1,curve 3)exhibited a maximum and increased smoothly up to 22%at 280?C and then it decreased to 4%at 320?C and remained at this value over the temperatures studied.Gas started to appear at 260?C and gradually reached 6%at 350?C and 1h.From these results,a simpli?ed reaction pathway to residue particles or char must occur as:
glucose →water solubles (5-HMF ,levoglucosan)→oil +residue
(1)
Fig.8.Reaction paths for the production of particles,gas and oil from decomposition of glucose under hydrothermal conditions.
Fig.7.UV/vis absorbance spectra when glucose solution was heated to 350?C at 0.2?C/s (lines denote,1–5:measurements after cooling from 250,320,350?C,{350?C +5min }and {350?C +10min };0:standard initial glucose solution;6:aque-ous solution of caramelized glucose by heating solid glucose to 160?C for 15min).
oil →residue →char
(2)
where reactions (1)and (2)occur in parallel.3.2.Diamond anvil cell experiments
In the DAC experiments,aqueous glucose solutions were reacted at much higher pressures (ca.180MPa)than for the autoclave experiments.High pressures can promote [41,42]or suppress reac-tions so some discussion of the effects is appropriate.Aida et al.[41]studied hydrothermal reaction of glucose at high temperature and found that increasing pressure from 40to 80MPa enhanced the dehydration reactions to 5-HMF,and also enhanced hydroly-sis of 5-HMF leading to the production of 1,2,4-benzenetriol.On the other hand,for the cases where gases are formed,it is possi-ble that high pressures can suppress gasi?cation rates due to Le Chatelier’s principle.Due to the tiny product amounts in the DAC,
Z.Fang et al./J.of Supercritical Fluids56 (2011) 41–4745
only micro-spectroscopic(e.g.,IR or Raman microscopy)analyses of product residues can be conducted and these are limited to qual-itative measurements,since the O–H and C–H vibrations can be assigned to many types of compounds(e.g.,alcohols,sugars and hydrocarbons).Analyses of the gases formed are not possible with our setup although specialized techniques do exist in the litera-ture[48].The IR spectra presented give qualitative changes of the decomposed glucose products as compared with the standard glu-cose spectrum.
3.2.1.Slow heating experiments
The same heating rate as that used for the autoclave(0.18?C/s) was used in the DAC experiments observed with transmitted optical light.In Fig.2,aqueous glucose with gas bubbles was slowly heated to350?C and136MPa.At220?C,the solution color changed to light yellow(Fig.2b),indicating glucose conversion to 5-HMF and levoglucosan(Fig.1,curves4–6)that were immediately polymerized to colored solutions via caramelization.Gas bubbles disappeared and the solution changed to an orange color along with the precipitation of numerous particles at251?C(Fig.2d).The orange color became more brilliant as further particles precipitated (Fig.2e).At280?C(Fig.2f),the entire chamber was practically?lled with black particles that seemed to become slightly denser as tem-perature increased further to350?C(Fig.2f–j).After reaction,an orange?lm was formed on the upper diamond anvil,and parti-cle residue remained on the lower anvil(supplementary material Fig.S1).IR analyses of the upper(Fig.5d up)and lower(Fig.5d low) anvils were made.The lower anvil(Fig.5d low)results showed that the particle residue decomposed but still had a glucose-like character as can be seen that it remained three broad character-istic peaks at around2932cm?1and had an additional peak at 1608cm?1,as compared with the standard glucose spectrum.The oil-?lm(Fig.5d up)had little glucose-like character since no peaks were present at around2932cm?1.The solid residue was ana-lyzed by SEM which showed that sub-micron size particles were formed(Fig.4,100nm).Images of the residues obtained from262 to350?C showed that yellow or orange oil-like?lms were formed in1320–1800s reaction time on the upper anvil,while solid parti-cles precipitated on the lower anvil at temperatures above262?C. IR spectra revealed that all the precipitated particles were glucose-like with a similar chemical structure as that of the residue d low (Fig.5).
3.2.2.Rapid heating experiments
Aqueous glucose solutions were subjected to rapid heating was studied(ca.9.5–17.0?C/s)to410?C and observed with microscopy. In Fig.3,aqueous glucose was heated to410?C and181MPa(heat-ing rate,14.3?C/s;water density,768kg/m3).Little changes in the solution appearance occurred at temperatures up to280?C(Fig.3c) besides the disappearance of the gas bubbles due to the expan-sion of the liquid water phase.The solution color changed from clear to light yellow at300?C and then to dark yellow which deep-ened in color at320?C(Fig.3d–e).There was little change in the deep yellow color until400?C,at which numerous particles pre-cipitated along with the appearance of an orange color(Fig.3e–i). After the chamber was cooled from its maximum temperature of 410?C(Fig.3j),yellow sugar-like residues were found on the lower diamond anvil(supplementary material Fig.S2).IR results showed that the residues had more glucose-like character than the particle residues that were formed during slow heating experiments even at the lower temperatures(350?C)as shown by the remaining peak at 1454cm?1(Fig.6c vs.Fig.5d low and1).Colorful sugar-like residues and black particles were obtained on the lower anvil that were formed by rapid heating(9.5–17?C/s)to320–410?C in17–44s and medium heating(4.6?C/s)to410?C in820s reaction time.As tem-perature increased to410?C,the residue color changed from red at 320?C,to brown at360?C and then to yellow at410?C,whereas the medium heating rate to410?C gave black particles and a black ?lm by pyrolysis(supplementary material Fig.S2).All the residues had similar chemical structures as glucose(Fig.6).It is possible that the colorful sugar-like residues that formed below410?C could be used as food colorants due to their appearance and stability relative to the other residues.
When aqueous glucose solutions are heated at slow heating rates,solid particles form at very low temperatures which then undergo heterogeneous decomposition to aromatic char[18].How-ever,when the solutions remained as a homogenous phase for the case of rapid heating rates up to380?C as studied in this work,lit-tle char was formed.This phenomenon was observed by Modell and co-workers in the1970s,who found that at supercritical water conditions,little char and tar were found and the glucose solu-tions became gasi?ed[49–51].In the work of Chuntanapum and Matsumura for the decomposition of5-HMF(0.15M for3000s),no char particles were detectable under supercritical conditions,but particles could be observed under subcritical conditions[52].
The color change of glucose solution during heating to high tem-perature is similar to the well-known caramelization that is one of the more important types of browning processes in food sci-ence.However,the initial caramelization of glucose starts at much lower temperatures of160?C,after condensation,isomerization, dehydration and fragmentation reactions(?avor production)occur, and colors are produced by polymerization to various high molecu-lar weight components(e.g.,caramelans:C24H36O18;caramelens: C36H50O25;caramelins:C125H188O80)[53,54].We measured UV/vis absorbance spectra(Shimadzu,UV-1800)of the decomposed glu-cose solutions after they were cooled from different temperatures (up to350?C and holding for10min)(Fig.7).It was found that the wavelength shifted to higher values when reaction tempera-ture increased to350?C and was held for5min at those conditions (Fig.7,lines0–4),but shifted to lower wavelengths and the solu-tion color became lighter at350?C as the holding time increased to 10min(Fig.7,line5vs.4).The possible reason for this phenomenon is that colored solutions further polymerized and then precipi-tated as char particles such that concentrations would be reduced for long reaction times or higher temperatures.Chuntanapum and Matsumura[52,55]found that glucose produces char particles2 orders of magnitude faster than5-HMF feedstock.The interaction of5-HMF with the other glucose decomposition products seems to play an important role in the production of char.They proposed simpli?ed formation pathways of char particles:char was formed from the decomposed products of furfural,5-HMF and other water soluble products[55].
Based on the above discussions and our previous work[18], reaction paths are proposed for the production of dyes,particles, gas and oil(Fig.8):colorful dye-like substances are produced via caramelization upon both slow and rapid-heating;char particles are produced by non-catalytic decomposition of glucose to aque-ous products(including furfurals,5-HMF,levoglucosan and colored materials),and subsequent re-polymerization during slow heating up to350?C or rapid heating to higher temperatures.The particles are submicron or nano-sized biochar with aromatic structures[18], and these are dif?cult to convert further to gas and oil at low tem-peratures(<400?C).A high heating rate can be used to produce dyes and to avoid further polymerization of products to char particles. For the case of heating the aqueous glucose solution with catalysts to high temperatures,gas(with Ni,Ni–Sn,Pt/Al2O3)[18,56,57],oil (with Na2CO3)[18],liquid alkanes(with Pt/SiO2–Al2O3)[58]and chemicals(with Pt/?-Al2O3)[59]can be produced under homoge-neous conditions.
From the above experimental results,it can be seen that dyes or particles can be produced by non-catalytic hydrothermal decom-position of glucose.The glucose required for dyes can be produced
46Z.Fang et al./J.of Supercritical Fluids56 (2011) 41–47
from renewable resources by simultaneous hydrolysis of cellulose or wood under hydrothermal conditions[14,37,60].The micro-particles can possibly?nd applications as fertilizers,support for catalysts,activated carbon,explosives and composite materials. Recently,Peterson et al.[61]demonstrated that adding glycine increased or decreased brown colors and characteristic odors asso-ciated with the Maillard reaction,which can be used for the production of dyes and particles.The results presented here show the nature of the homogeneous reaction conditions and its impor-tance for converting glucose into products of dyes,particles,oil and gases.
4.Conclusions
When aqueous glucose solutions are slowly heated to350?C,the solutions change color from clear to light yellow,and then to yellow and?nally to orange.Aqueous glucose solutions begin decompos-ing at220?C,and particles precipitate at251?C.At280?C with slow heating,particles readily form.At350?C with slow heating,aque-ous glucose solutions react to form65%solid residue that consist of nanoparticles can be obtained as identi?ed by SEM.
When aqueous solutions of glucose are heated rapidly,they remain homogenous until about380?C,and colorful materials are produced with little particle precipitation up to410?C.From these ?ndings,the heating process can be used to produce dyes and catalytically gasify and liquefy glucose or synthesize chemicals under a homogeneous reaction environment.The appearance of the reacting phase of glucose in water can be used to control product distribution and even the nature of the chemical products.
Slow heating of aqueous glucose solutions produce particles at the sub-micron or possibly nanometer order that have aromatic structures,which is most likely due to condensation reactions involving5-HMF.The aromatic structures have the potential use as activated carbon,support for catalysts,and in energetic materi-als and composites.Rapid heating rates lead to sugar-like products that are yellow and then become orange depending on the condi-tions.These orange like compounds can be possibly used as dyes or further decomposed into oil and gas with catalysts.
In the reaction of aqueous glucose solutions,it is possible to monitor the progress of the reaction through color changes if opti-cal access is available.In the reaction of glucose in high temperature water,color or UV/Vis spectra can be possibly used to monitor or control the products formed or the product distribution.The glu-cose decomposition products may have use as colorants in the food industry or as additives in the printing and coating industries. Acknowledgments
The author(ZF)wishes to acknowledge the?nancial support from Chinese Academy of Sciences(Bairenjihua,knowledge inno-vation key project),and China National Natural Science Foundation. The authors wish to thank Ph.D.candidate,Mr.Y.D.Long for con-ducting the UV/vis analyses.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at doi:10.1016/j.sup?u.2010.11.009. References
[1]A.Kruse,E.Dinjus,Hot compressed water as reaction medium and reactant:
properties and synthesis reactions,J.Supercrit.Fluids39(2007)362–380. [2]A.Kruse,E.Dinjus,Hot compressed water as reaction medium and reactant:2.
Degradation reactions,J.Supercrit.Fluids41(2007)361–379.
[3]M.Uematsu,E.U.Franck,Static dielectric constant of water and steam,J.Phys.
Chem.Ref.Data9(1980)1291–1306.
[4]W.L.Marshall,E.U.Franck,Ion product of water substance,0–1000?C,1–10,000
bars new international formulation and its background,J.Phys.Chem.Ref.Data 10(1981)295–304.
[5]J.W.Tester,H.R.Holgate,F.J.Armellini,P.A.Webley,W.R.Killilea,G.T.Hong,
H.E.Barner,Supercritical water oxidation technology:process development
and fundamental research,ACS Symp.Ser.518(1993)35–76.
[6]R.W.Shaw,N.Dahmen,Destruction of toxic organic materials using supercriti-
cal water oxidation:current state of the technology,NATO Sci.Ser.E:Appl.Sci.
366(2000)425–437.
[7]Z.Fang,J.A.Kozinski,Phase changes of benzo(a)pyrene in supercritical water
combustion,Combust.Flame124(2001)255–267.
[8]Z.Fang,Rapid Production of Micro-and Nano-particles using Supercritical
Water,Springer-Verlag,Heidelberg,2010.
[9]P.E.Savage,Organic chemical reactions in supercritical water,Chem.Rev.99
(1999)603–621.
[10]J.N.Chheda,G.W.Huber,J.A.Dumesic,Liquid-phase catalytic processing of
biomass-derived oxygenated hydrocarbons to fuels and chemicals,Angew.
Chem.Int.Ed.46(2007)7164–7183.
[11]M.Sasaki,T.Adschiri,K.Arai,Production of cellulose II from native cellulose
by near-and supercritical water solubilization,J.Agric.Food Chem.51(2003) 5376–5381.
[12]Z.Fang,C.Fang,Complete dissolution and hydrolysis of wood in hot water,
AIChE J.54(2008)2751–2758.
[13]O.Bobleter,Hydrothermal degradation of polymers derived from plants,Prog.
Polym.Sci.19(1994)797–841.
[14]M.Sasaki,Z.Fang,Y.Fukushima,T.Adschiri,K.Arai,Dissolution and hydrolysis
of cellulose in subcritical and supercritical water,Ind.Eng.Chem.Res.39(2000) 2883–2890.
[15]M.J.Antal Jr.,S.G.Allen,D.Schulman,X.Xu,R.J.Divilio,Biomass gasi?cation in
supercritical water,Ind.Eng.Chem.Res.39(2000)4040–4053.
[16]M.Osada,T.Sato,M.Watanabe,M.Shirai,K.Arai,Catalytic gasi?cation of
wood biomass in subcritical and supercritical water,Combust.Sci.Technol.
178(2006)537–552.
[17]Y.J.Lu,L.J.Guo,C.M.Ji,X.M.Zhang,X.H.Hao,Q.H.Yan,Hydrogen production by
biomass gasi?cation in supercritical water:a parametric study,Int.J.Hydrogen Energy31(2006)822–831.
[18]Z.Fang,T.Minowa,R.L.Smith Jr.,T.Ogi,J.A.Kozinski,Liquefaction and gasi?-
cation of cellulose with Na2CO3and Ni in subcritical water at350?C,Ind.Eng.
Chem.Res.43(2004)2454–2463.
[19]M.Modell,Gasi?cation and liquefaction of forest products in supercritical
water,in:R.P.Overend,https://www.doczj.com/doc/6a13542739.html,ne,L.K.Mudge(Eds.),Fundamentals of Ther-mochemical Biomass Conversion,Elsevier Appl.Sci.,London,UK,1985,pp.
95–119.
[20]G.W.Huber,S.Iborra,A.Corma,Synthesis of transportation fuels from biomass:
chemistry,catalysts,and engineering,Chem.Rev.106(2006)4044–4098. [21]D.Yu,M.Aihara,M.J.Antal Jr.,Hydrogen production by steam reforming glucose
in supercritical water,Energy Fuels7(1993)574–577.
[22]M.Watanabe,H.Inomata,K.Arai,Catalytic hydrogen generation from biomass
(glucose and cellulose)with ZrO2in supercritical water,Biomass Bioenergy22 (2002)405–410.
[23]X.Xu,Y.Matsumura,J.Stenberg,M.J.Antal Jr.,Carbon-catalyzed gasi?cation
of organic feedstocks in supercritical water,Ind.Eng.Chem.Res.35(1996) 2522–2530.
[24]H.Schmieder,J.Abeln,N.Boukis,E.Dinjus,A.Kruse,M.Kluth,G.Petrich,E.
Sadri,M.Schacht,Hydrothermal gasi?cation of biomass and organic wastes,J.
Supercrit.Fluids17(2000)145–153.
[25]A.Sinag,A.Kruse,J.Rathert,In?uence of the heating rate and type of catalyst
on the formation of key intermediates and on the generation of gases during hydropyrolysis of glucose in supercritical water in a batch reactor,Ind.Eng.
Chem.Res.43(2004)502–508.
[26]R.Hashaikeh,Z.Fang,I.S.Butler,J.A.Kozinski,Sequential hydrothermal
gasi?cation of biomass to hydrogen,https://www.doczj.com/doc/6a13542739.html,bust.Inst.30(2005)2231–2237.
[27]P.T.Williams,J.Onwudili,Composition of products from the supercritical water
gasi?cation of glucose:a model biomass compound,Ind.Eng.Chem.Res.44 (2005)8739–8749.
[28]P.T.Williams,J.Onwudili,Subcritical and supercritical water gasi?cation
of cellulose,starch,glucose,and biomass waste,Energy Fuels20(2006) 1259–1265.
[29]A Sinag,A.Kruse,V.Schwarzkopf,Key compounds of the hydropyrolysis of
glucose in supercritical water in the presence of K2CO3,Ind.Eng.Chem.Res.42 (2003)3516–3521.
[30]X.H.Hao,L.J.Guo,X.Mao,X.M.Zhang,X.J.Chen,Hydrogen production from
glucose used as a model compound of biomass gasi?ed in supercritical water, Int.J.Hydrogen Energy28(2003)55–64.
[31]M.Sasaki,K.Goto,K.Tajima,T.Adschiri,K.Arai,Rapid and selective retro-aldol
condensation of glucose to glycolaldehyde in supercritical water,Green Chem.
4(2002)285–287.
[32]I.-G.Lee,M.-S.Kim,S.-K.Ihm,Gasi?cation of glucose in supercritical water,Ind.
Eng.Chem.Res.41(2002)1182–1188.
[33]M.Watanabe,F.Bayer,A.Kruse,Oil formation from glucose with formic
acid and cobalt catalyst in hot-compressed water,Carbohydr.Res.341(2006) 2891–2900.
[34]B.M.Kabyemela,T.Adschiri,R.M.Malaluan,K.Arai,H.Ohzeki,Rapid and selec-
tive conversion of glucose to erythrose in supercritical water,Ind.Eng.Chem.
Res.36(1997)5063–5067.
Z.Fang et al./J.of Supercritical Fluids56 (2011) 41–4747
[35]B.M.Kabyemela,T.Adschiri,R.M.Malaluan,K.Arai,Kinetics of glucose epimer-
ization and decomposition in subcritical and supercritical water,Ind.Eng.
Chem.Res.36(1997)1552–1558.
[36]B.M.Kabyemela,T.Adschiri,R.M.Malaluan,K.Arai,Glucose and fructose
decomposition in subcritical and supercritical water:detailed reaction path-way,mechanisms,and kinetics,Ind.Eng.Chem.Res.38(1999)2888–2895. [37]M.Sasaki,B.M.Kabyemela,R.M.Malaluan,S.Hirose,N.Takeda,T.Adschiri,
K.Arai,Cellulose hydrolysis in subcritical and supercritical water,J.Supercrit.
Fluids13(1998)261–268.
[38]Z.Fang,T.Minowa,C.Fang,R.L.Smith Jr.,H.Inomata,J.A.Kozinski,Catalytic
hydrothermal gasi?cation of cellulose and glucose,Int.J.Hydrogen Energy33 (2008)981–990.
[39]Y.Roman-Leshkov,C.J.Barrett,Z.Y.Liu,J.A.Dumesic,Production of dimethyl-
furan for liquid fuels from biomass-derived carbohydrates,Nature447(2007) 982–985.
[40]H.Zhao,J.E.Holladay,H.Brown,Z.C.Zhang,Metal chlorides in ionic liq-
uid solvents convert sugars to5-hydroxymethylfurfural,Science316(2007) 1597–1600.
[41]T.M.Aida,Y.Sato,M.Watanabe,K.Tajima,T.Nonaka,H.Hattori,K.Arai,Dehy-
dration of d-glucose in high temperature water at pressures up to80MPa,J.
Supercrit.Fluids40(2007)381–388.
[42]T.M.Aida,K.Tajima,M.Watanabe,Y.Saito,K.Kuroda,T.Nonaka,H.Hat-
tori,R.L.Smith Jr.,K.Arai,Reactions of d-fructose in water at temperatures up to400?C and pressures up to100MPa,J.Supercrit.Fluids42(2007)110–119.
[43]M.Wang,J.Z.Yang,Study of water-soluble sulfur black dye containing glucose
groups,J.Dalian Univ.Sci.Technol.42(2002)428–430(in Chinese).
[44]C.Yao,Y.Shin,L.Q.Wang,C.F.Windisch Jr.,W.D.Samuels,B.W.Arey,C.Wang,
W.M.Risen Jr.,G.J.Exarhos,Hydrothermal dehydration of aqueous fructose solutions in a closed system,J.Phys.Chem.C111(2007)15141–15145. [45]T.Minowa,Z.Fang,Hydrogen production from cellulose in hot compressed
water using reduced nickel catalyst:products distribution at different reaction temperatures,J.Chem.Eng.Jpn.31(1998)488–491.
[46]W.A.Bassett,A.H.Shen,M.Bucknum,I.-M.Chou,A new diamond anvil cell for
hydrothermal studies to2.5GPa and from-190to1200?C,Rev.Sci.Instrum.64 (1993)2340–2345.[47]R.L.Smith Jr.,Z.Fang,Techniques,applications and future prospects of diamond
anvil cells for studying supercritical water systems,J.Supercrit.Fluids47(2009) 431–446.
[48]T.Azbej,M.J.Severs,B.G.Rusk,R.J.Bodnar,In situ quantitative analysis of indi-
vidual H2O–CO2?uid inclusions by laser Raman spectroscopy,Chem.Geol.237 (2007)255–263.
[49]M.Modell,R.C.Reid,S.I.Amin,Gasi?cation process,US Patent4,113,446(1978).
[50]M.Modell,Reforming of glucose and wood at the critical conditions of water,
ASME(Paper)(1977)(77-ENAs-2).
[51]S.Amin,R.C.Reid,M.Modell,Reforming and decomposition of glucose in an
aqueous phase,ASME(Paper)(1975)(75-ENAs-21).
[52]A.Chuntanapum,Y.Matsumura,Formation of tarry material from5-HMF in
subcritical and supercritical water,Ind.Eng.Chem.Res.48(2009)9837–9846.
[53]https://www.doczj.com/doc/6a13542739.html,/uk/colour/caramel.htm.
[54]B.Jiang,Y.T.Liu,B.Bhandari,W.B.Zhou,Impact of caramelization on the glass
transition temperature of several caramelized sugars.Part I,chemical analyses, J.Agric.Food Chem.56(2008)5138–5147.
[55]A.Chuntanapum,Y.Matsumura,Char formation mechanism in supercritical
water gasi?cation process:a study of model compounds,Ind.Eng.Chem.Res.
49(2010)4055–4062.
[56]G.W.Huber,J.W.Shabaker,J.A.Dumesic,Raney Ni–Sn catalyst for H2production
from biomass-derived hydrocarbons,Science300(2003)2075–2077.
[57]R.D.Cortright,R.R.Davsa,J.A.Dumsic,Hydrogen from catalytic reform-
ing of biomass-derived hydrocarbon in liquid water,Nature418(2002) 964–967.
[58]G.W.Huber,J.N.Chheda,C.J.Barrett,J.A.Dumesic,Production of liquid alkanes
by aqueous-phase processing of biomass-derived carbohydrates,Science308 (2005)1446–1450.
[59]A.Fukuoka,P.L.Dhepe,Catalytic conversion of cellulose into sugar alcohols,
Angew.Chem.Int.Ed.45(2006)5161–5163.
[60]Z.Fang,Noncatalytic fast hydrolysis of wood,Bioresour.Technol.,in press,
doi:10.1016/j.biortech.2010.10.063.
[61]A.A.Peterson,https://www.doczj.com/doc/6a13542739.html,chance,J.W.Tester,Kinetic evidence of the Maillard
reaction in hydrothermal biomass processing:glucose–glycine interactions in high-temperature,high-pressure water,Ind.Eng.Chem.Res.49(2010) 2107–2117.
of与for的用法以及区别 for 表原因、目的 of 表从属关系 介词of的用法 (1)所有关系 this is a picture of a classroom (2)部分关系 a piece of paper a cup of tea a glass of water a bottle of milk what kind of football,American of soccer? (3)描写关系 a man of thirty 三十岁的人 a man of shanghai 上海人 (4)承受动作 the exploitation of man by man.人对人的剥削。 (5)同位关系 It was a cold spring morning in the city of London in England. (6)关于,对于 What do you think of Chinese food? 你觉得中国食品怎么样? 介词 for 的用法小结 1. 表示“当作、作为”。如: I like some bread and milk for breakfast. 我喜欢把面包和牛奶作为早餐。What will we have for supper? 我们晚餐吃什么?
2. 表示理由或原因,意为“因为、由于”。如: Thank you for helping me with my English. 谢谢你帮我学习英语。 Thank you for your last letter. 谢谢你上次的来信。 Thank you for teaching us so well. 感谢你如此尽心地教我们。 3. 表示动作的对象或接受者,意为“给……”、“对…… (而言)”。如: Let me pick it up for you. 让我为你捡起来。 Watching TV too much is bad for your health. 看电视太多有害于你的健康。 4. 表示时间、距离,意为“计、达”。如: I usually do the running for an hour in the morning. 我早晨通常跑步一小时。We will stay there for two days. 我们将在那里逗留两天。 5. 表示去向、目的,意为“向、往、取、买”等。如: let’s go for a walk. 我们出去散步吧。 I came here for my schoolbag.我来这儿取书包。 I paid twenty yuan for the dictionary. 我花了20元买这本词典。 6. 表示所属关系或用途,意为“为、适于……的”。如: It’s time for school. 到上学的时间了。 Here is a letter for you. 这儿有你的一封信。 7. 表示“支持、赞成”。如: Are you for this plan or against it? 你是支持还是反对这个计划? 8. 用于一些固定搭配中。如: Who are you waiting for? 你在等谁? For example, Mr Green is a kind teacher. 比如,格林先生是一位心地善良的老师。
从电视剧到大电影 门快速增长的产业 3 月8 日是星期日,王府井步行街上行人如潮,对忙碌 了一周的年轻上班族来说,通常的周末度过方式就是一逛街、 馆子,然后看场电影。从坐落在步行街起点的东方新天地经过 王府井百货走到步行街终点的新东安APM 购物中心的 800 米路程范围内,就有3 座多厅影院,影院门口聚集着等待 入场的年轻男女。 而在王府井步行街终点再往北700 米的一家五星级酒店 中,一场电影新片发布会正在紧锣密鼓地进行中,绚丽的灯光照亮 着大厅的前台,在主持人的解说和观众的欢呼声中,几位主演依次 上台,高圆圆戴着印着姜武头像的拳击手套现场表演健身拳,刘涛 制作了一个面包,郭碧婷则玩了一个盲摸箱子里的小动物的游戏。 第二天,人们会在各大报纸和门 户网站的娱乐版发现电影《咱们结婚吧》发布会的新闻和现 场图片,接下来的宣传会不断放出影片的资料,直到4月2 日电影上映。 每个周末,娱乐记者们都会奔波在不同电影发布会的现
场―经过数年的高速增长之后,中国电影产业已经成为全世 界瞩目的焦点。刚刚过去的105 天“贺岁档中国电影市场共产出了107亿人民币的票房,平均每天超过 1 个亿。而在2 月份,中国电影一个月内产出41 亿元人民币票房,超过了同期美国市场月度票房。腾讯电影频道主编曾剑在微信 公众号人间电影指南”中乐观地写道:“春节档能捞20 亿,说明国富民强,人民安居乐业,有闲钱看电影,有时间看 电影;二说明中国的电影产业路数越来越像样了,它越来越像 个巨大的电影市场该有的那个样子、该有的那些类型、该有的那些制作模式。接下来,应该把它做得更精致一些,更好看些。” 电影产业高速发展的另一面则是好故事的稀缺,据电影 网站时光网统计,2013 年国产电影票房前十名时光网平均分 为6.32 ,而2014 年前十名的平均得分下降到5.88;在去年北京电影节“中外电影合作论坛”上,万达文化副总裁叶宁就 表达过一个观点:当下中国电影最重要的是“去找到好的故事,好的人来合作。 个扎实的电影剧本 46 岁的刘江面色红润,圆寸和头型融为一体,身材有些发福,看上去有点像小一号的弥勒佛,很难让人相信他曾经是话剧院的演员、电影学院表演系的毕业生。刘江的经历有点像李安,电影学院毕业后,他做过驻唱歌手,后来又在家
to与for的用法和区别 一般情况下, to后面常接对象; for后面表示原因与目的为多。 Thank you for helping me. Thanks to all of you. to sb.表示对某人有直接影响比如,食物对某人好或者不好就用to; for表示从意义、价值等间接角度来说,例如对某人而言是重要的,就用for. for和to这两个介词,意义丰富,用法复杂。这里仅就它们主要用法进行比较。 1. 表示各种“目的” 1. What do you study English for? 你为什么要学英语? 2. She went to france for holiday. 她到法国度假去了。 3. These books are written for pupils. 这些书是为学生些的。 4. hope for the best, prepare for the worst. 作最好的打算,作最坏的准备。 2.对于 1.She has a liking for painting. 她爱好绘画。 2.She had a natural gift for teaching. 她对教学有天赋/ 3.表示赞成同情,用for不用to. 1. Are you for the idea or against it? 你是支持还是反对这个想法? 2. He expresses sympathy for the common people.. 他表现了对普通老百姓的同情。 3. I felt deeply sorry for my friend who was very ill. 4 for表示因为,由于(常有较活译法) 1 Thank you for coming. 谢谢你来。 2. France is famous for its wines. 法国因酒而出名。 5.当事人对某事的主观看法,对于(某人),对…来说(多和形容词连用)用介词to,不用for.. He said that money was not important to him. 他说钱对他并不重要。 To her it was rather unusual. 对她来说这是相当不寻常的。 They are cruel to animals. 他们对动物很残忍。 6.for和fit, good, bad, useful, suitable 等形容词连用,表示适宜,适合。 Some training will make them fit for the job. 经过一段训练,他们会胜任这项工作的。 Exercises are good for health. 锻炼有益于健康。 Smoking and drinking are bad for health. 抽烟喝酒对健康有害。 You are not suited for the kind of work you are doing. 7. for表示不定式逻辑上的主语,可以用在主语、表语、状语、定语中。 1.It would be best for you to write to him. 2.The simple thing is for him to resign at once. 3.There was nowhere else for me to go. 4.He opened a door and stood aside for her to pass.
For的用法 1. 表示“当作、作为”。如: I like some bread and milk for breakfast. 我喜欢把面包和牛奶作为早餐。 What will we have for supper? 我们晚餐吃什么? 2. 表示理由或原因,意为“因为、由于”。如: Thank you for helping me with my English. 谢谢你帮我学习英语。 3. 表示动作的对象或接受者,意为“给……”、“对…… (而言)”。如: Let me pick it up for you. 让我为你捡起来。 Watching TV too much is bad for your health. 看电视太多有害于你的健康。 4. 表示时间、距离,意为“计、达”。如: I usually do the running for an hour in the morning. 我早晨通常跑步一小时。 We will stay there for two days. 我们将在那里逗留两天。 5. 表示去向、目的,意为“向、往、取、买”等。如: Let’s go for a walk. 我们出去散步吧。 I came here for my schoolbag.我来这儿取书包。 I paid twenty yuan for the dictionary. 我花了20元买这本词典。 6. 表示所属关系或用途,意为“为、适于……的”。如: It’s time for school. 到上学的时间了。 Here is a letter for you. 这儿有你的一封信。 7. 表示“支持、赞成”。如: Are you for this plan or against it? 你是支持还是反对这个计划? 8. 用于一些固定搭配中。如: Who are you waiting for? 你在等谁? For example, Mr Green is a kind teacher. 比如,格林先生是一位心地善良的老师。 尽管for 的用法较多,但记住常用的几个就可以了。 to的用法: 一:表示相对,针对 be strange (common, new, familiar, peculiar) to This injection will make you immune to infection. 二:表示对比,比较 1:以-ior结尾的形容词,后接介词to表示比较,如:superior ,inferior,prior,senior,junior 2: 一些本身就含有比较或比拟意思的形容词,如equal,similar,equivalent,analogous A is similar to B in many ways.
院线新片网络防盗有监测,426so平台为中国知识产权护航院线新片网络防盗有监测,426so平台为中国知识 产权护航 都市前沿 电影《失孤》3月20日上映第三天22时,出现首个可下载传播源。《王牌特工:特工学院》3月27日内地上映第三天,出现首个可下载传播源。《战狼》4月2日上映当天18时,出现首个可下载传播源。《咱们结婚吧》4月2日上映两天后,出现首个枪版在线播放传播源。《速度与激情7》4月3日美国上映当天,国内出现首个在线播放传播源,比内地上映时间提前9天――这是“中国国际知识产权维权监测平台”(426so平台)发布的第一组盗版检测数据。 4月22日,由中国音像与数字出版协会数字音像工作委员会发起的“中国国际知识产权维权监测平台(426so平台)发布会,中国国际影视版权监测维权座谈会”在北京举行。 中国电影著作权协会、中国广播电视协会有线电视工作委员会、中国广播电视协会电视制片委员会等行业组织代表;华谊兄弟娱乐、韩国希杰娱乐CHE&M、SOHU 视频、福建恒业、土豆网、1905电影网等百余家知名影视公司代表;美国电影协会,韩国电影振兴委员会、华纳兄弟、索尼影视等国外影视机构代表参加了本次活动。 中国国际知识产权监测维权平台,又称426so平台,是国家数字音像传播服务监管平台旗下子平台。据了解,426so平台基于国家数字音像传播服务监管平台前期工作成果,目前已形成针对解决方案,为各类版权人提供监测维权服务,提供作品传播监测、盗版预警、盗版源头追踪、侵权证据固定、损失评估、举报清除、诉讼维权等一系列服务。能够配合新片上映
时间,尽可能将网络盗版损失降至最低,保障票房收益最大化。 中国音像与数字出版协会副理事长兼数字音像工作委员会主任费玉珍说:“随着产业的健康良性发展,音像出版及视听产业将成为万亿产值的新产业,国家数字音像传播服务监管平台将始终如一为产业的发展保驾护航。” 在426so平台发布会上,主办方与参会的协会代表、影视机构等就中国影视盗版维权的现状进行了座谈,并倡议发起中国国际影视版权监测维权联盟。主办方则表示:影视版权领域的版权人、版权机构、行业协会、律师事务所、维权组织、知识产权机构等都可自愿加入联盟,联盟将通过426so平台为成员单位提供免费的监测报告。 李淳首秀《对风说爱你》,李安力挺儿子进军内地 近日,电影《对风说爱你》举行了“情书告白”发布会,金马奖导演王童携主演郭采洁、郭碧婷、胡宇威、柯佳?鳌?钕?川悉数到场。发布会现场华人导演李安惊喜“现身”,称导演王童是他的前辈,电影所演绎的动人爱情承接着台湾人宝贵的往事。李安儿子李淳在片中也有着重要的戏份。他提到在电影拍摄中为了更好地饰演角色,请教了很多前辈,前辈们也都称赞他是拥有正能量的“未来之星”。《对风说爱你》宣布定档6.19,晴天上映。 电影《枪过境》首映,讲述真实缉毒故事 4月15日,“电影《枪过境》首映式暨大银幕(北京)电影发行公司春季发布会”在人民大会堂举行。电影出品人、银幕(北京)电影发行控股有限公司董事长施建祥与大银幕电影发行控股有限公司总经理王??携《枪过境》、《三千里》、《上海王》、《叶问3》、《大轰炸》等电影主创人员亮相。由梁杰导演的《枪过境》改编自基层民警的真实故事,讲述了内蒙边境警察与毒贩斗智斗勇的历险故事。 “创?纪录运动”抢戏北京国际电影节
of 1....的,属于 One of the legs of the table is broken. 桌子的一条腿坏了。 Mr.Brown is a friend of mine. 布朗先生是我的朋友。 2.用...做成的;由...制成 The house is of stone. 这房子是石建的。 3.含有...的;装有...的 4....之中的;...的成员 Of all the students in this class,Tom is the best. 在这个班级中,汤姆是最优秀的。 5.(表示同位) He came to New York at the age of ten. 他在十岁时来到纽约。 6.(表示宾格关系) He gave a lecture on the use of solar energy. 他就太阳能的利用作了一场讲演。 7.(表示主格关系) We waited for the arrival of the next bus. 我们等待下一班汽车的到来。
I have the complete works of Shakespeare. 我有莎士比亚全集。 8.来自...的;出自 He was a graduate of the University of Hawaii. 他是夏威夷大学的毕业生。 9.因为 Her son died of hepatitis. 她儿子因患肝炎而死。 10.在...方面 My aunt is hard of hearing. 我姑妈耳朵有点聋。 11.【美】(时间)在...之前 12.(表示具有某种性质) It is a matter of importance. 这是一件重要的事。 For 1.为,为了 They fought for national independence. 他们为民族独立而战。 This letter is for you. 这是你的信。
《小时代4》经典台词 《小时代4:灵魂尽头》改编自郭敬明小说《小时代》,由郭敬明担任影片的编剧及导演,李力制片,杨幂、郭采洁、陈学冬、郭碧婷、谢依霖主演。该片是《小时代》系列电影的第四部,影片围绕林萧、顾里、南湘、唐宛如四姐妹展开,讲述了顾里癌症、顾源坐牢、姐妹反目及这一群人的友谊方向是如何发展的的各种故事。> 《小时代4》台词欣赏> 血肉横飞只是开始而已。魂飞魄散才是真正的好戏。当然,我们都知道,我们热爱生活中这样刺激有跌宕的drama。> 一个人身边的位置只有那么多,你能给的也只有那么多,在这个狭小的圈子里,有些人要进来,就有一些人不得不离开。> 也许我们最终都会成长为自己曾经最讨厌的模样,但在此之前,面对黑暗无边,让我与你并肩。> 夕阳的光线像是被风吹散一般迅速消失,正如同再也回不去的美好年华。那感觉,像是一个时代最后的剧中。> 生活就是不可抗力,它就是合约里唯一一条、也是永远都会存在的一条无人可以更改的霸王条款。> 旋转着的,五彩缤纷的物质世界。等价交换的,最残酷的也最公平的寒冷人间。> 时间一点一滴地过去,流逝告别。我们慢慢地走向一个被上帝作记号的地点。> 但是生活永远不是连续剧。它不会再应该浪漫的时候,响起煽情的音乐;它不会再男主角深情告白的时候,就让女主角浓烈的回应;它不会再这样需要温柔和甜蜜的时刻,就打翻一杯浓浓的蜂蜜。> 后来,我的梦境总是反复出现这场无声无息的火,无数飞虫朝它飞去,它们仿佛是早就存在于这个世界的记忆碎片,旧时尘埃。> 阴影,都是死神某一个局部的轮廓,当太阳旋转到某一个角度,这些阴影,就会拼成一个完整的高举镰刀的英雄,那是我们就将一起挽手,走向灵魂的尽头。> 我们活在浩瀚的宇宙里,漫天漂浮的宇宙尘埃和星河光尘,我们是比这些还要渺小的存在。你并不知道生活在什么时候突然改变方向,陷入墨水一般浓稠的黑暗里去。你被失望拖进深渊,你被疾病拉进坟墓,你被挫折践踏的体无完肤,你被嘲笑、被讽刺、被讨厌、被怨恨、被放弃。但是我们却总在内心里保留着希望保留着不甘心放弃的心。我们依然在大大的绝望里小小的努力着。这种不想放弃的心情,它们变成无边黑暗的小小星辰。我们都是小小的星辰。> 如果我们的生活充满了以前另一种未知的可能性的话,那么在大学围墙范围内,这一场追逐大战,谁先遇到谁,都可以导致完全不同的结局。这就像有人在转盘里撒下一大把钢珠,在转盘没有停下来之前,谁都不知道最后的赢家会是谁。 《小时代》系列经典台词双语版 我用青春十年,赴你最后之约。 I bided ten years of my prime for our date with destiny. 这是最好的时代,也是最坏的时代。你会忘记这个时代,但你,会永远记住我们。 This is the best time, but the worst as well. You will eventually forget this day and this age, but you will always remember us.
1.两者都可以引出间接宾语,但要根据不同的动词分别选用介词to 或for:(1) 在give, pass, hand, lend, send, tell, bring, show, pay, read, return, write, offer, teach, throw 等之后接介词to。 如: 请把那本字典递给我。 正:Please hand me that dictionary. 正:Please hand that dictionary to me. 她去年教我们的音乐。 正:She taught us music last year. 正:She taught music to us last year. (2) 在buy, make, get, order, cook, sing, fetch, play, find, paint, choose,prepare, spare 等之后用介词for 。如: 他为我们唱了首英语歌。 正:He sang us an English song. 正:He sang an English song for us. 请帮我把钥匙找到。 正:Please find me the keys. 正:Please find the keys for me. 能耽搁你几分钟吗(即你能为我抽出几分钟吗)? 正:Can you spare me a few minutes? 正:Can you spare a few minutes for me? 注:有的动词由于搭配和含义的不同,用介词to 或for 都是可能的。如:do sb a favour=do a favour for sb 帮某人的忙 do sb harm=do harm to sb 对某人有害
for的用法: 1. 表示“当作、作为”。如: I like some bread and milk for breakfast. 我喜欢把面包和牛奶作为早餐。 What will we have for supper? 我们晚餐吃什么? 2. 表示理由或原因,意为“因为、由于”。如: Thank you for helping me with my English. 谢谢你帮我学习英语。 Thank you for your last letter. 谢谢你上次的来信。 Thank you for teaching us so well. 感谢你如此尽心地教我们。 3. 表示动作的对象或接受者,意为“给……”、“对…… (而言)”。如: Let me pick it up for you. 让我为你捡起来。 Watching TV too much is bad for your health. 看电视太多有害于你的健康。 4. 表示时间、距离,意为“计、达”。如:
I usually do the running for an hour in the morning. 我早晨通常跑步一小时。 We will stay there for two days. 我们将在那里逗留两天。 5. 表示去向、目的,意为“向、往、取、买”等。如: Let’s go for a walk. 我们出去散步吧。 I came here for my schoolbag.我来这儿取书包。 I paid twenty yuan for the dictionary. 我花了20元买这本词典。 6. 表示所属关系或用途,意为“为、适于……的”。如: It’s time for school. 到上学的时间了。 Here is a letter for you. 这儿有你的一封信。 7. 表示“支持、赞成”。如: Are you for this plan or against it? 你是支持还是反对这个计划? 8. 用于一些固定搭配中。如:
本文部分内容来自网络整理,本司不为其真实性负责,如有异议或侵权请及时联系,本司将立即删除! == 本文为word格式,下载后可方便编辑和修改! == 咱们结婚吧经典台词|经典语录|对白|句子|语句|片 段|桥段 《咱们结婚吧》讲述了四对即将举行婚礼的情侣距离婚前结婚一周时间, 因为婚前恐惧发生的各种冲突、逆转的故事。那么你知道咱们结婚吧经典台词 有哪些吗?下面是咱们结婚吧经典台词,欢迎查阅。 《咱们结婚吧》经典台词 1、人生就像减法,吃一顿少一顿,见一面少一面,珍惜你现在拥有的,错过了,就没了!眼前的一切就是最好的安排。 2、“我怕有别的男人,牵起你的手,为你带上婚戒。” 3、知道什么是优胜劣汰么?就是优质的女人剩下了,劣质的女人成为了别 人的太太! 4、眼前的安排就是最好的,命运会给人安排个最对的人。 5、“这份感情,会变成在我心中,最珍贵的记忆永存在心底。” 6、”一天当中,最美好的时刻,就是和喜欢的人在一起。” 7、女人一生之中,最美的时刻,就是穿上婚纱那一刻。 8、我怕我一辈子都见不到你。 9、就算有一百匹马,勒住我的脖子,我也会挣脱开,回来陪你去实现梦想。 10、如果你愿意,咱们结婚吧。 11、“我认识你两年的时间,我已暗恋了你七百三十天,我今天站在你面前,我是鼓了一百万次勇气才做到的。” 12、我想要有依靠想要有一个家有错吗? 13、“你是不是从来都没有想过要跟我结婚啊?”
14、婚姻是爱情的坟墓,不结婚,就死无葬身之地。 15、真正有感觉的、能携手终身的人,她不会从天上掉下来的,你不能站 在原地等待,你要主动地往前走,主动去寻找。是,这个过程当中,你一定会 遇到一些让自己伤心、难过、不开心的事情,你不用太在意,那些都是一个必 经的过程。走过它,你就会看见那个你真正有感觉的那个人。 16、“你长这么漂亮,肯定有好多人追你。” “谁追我啊,时间在追我。” 17、小子,为了你不再祸害人间乱搞男女关系,姐姐委屈点收了你吧,从 此记得鞍前马后随叫随到,不要挣扎了。 18、小子,你就从了大娘吧!把你的银行卡,信用卡,医疗保险,所有的卡,以及密码统统告诉我,让我帮你好好保管,包括你的人! 19、在这个世界上,任何事物都值得多看两眼。而眼前发生的一切,都是 最好的安排。 20、我可能不是最般配你的那个人,但我绝对是最爱你的那个人! 21、你别把自己当空气,你没那么重要,我是离不开空气,但是我离开的 了你。 22、北京的九月最美,因为九月的白天像八月,晚上像十月,就像我们三 十岁的女人既有二十岁女人的脸蛋,也有四十岁女人的智慧,这是女人一生之 中最美的时刻。 23、人的一生啊,总要碰上一些老天爷给的不公平的待遇,那么我们怎么 办呢?你不满、你抗议?你都不行的,没有用啊?既然已成事实了,咱们就勇敢的去面对它!过好我们的每一天。把我们这辈子的这日子过好它,你说好不好啊? 24、我知道当别人十分努力的时候,我只有二十分三十分的努力,才有可 能和那些十分努力的人一样。 25、我认识你两年的时间,我也暗恋了你七百三十天,我今天站在你面前,我是鼓了一百万次勇气才做到的。 26、女人一生之中最美的时刻,就是穿上婚纱的那一刻。 27、你不能站在原地去等待,你要往前走去寻找,是的,在这个过程中会 伤心,难过,这些都是必须经历的,走过它,你才会找到那个人。 28、从我认识你这天起,我恐婚的毛病逐渐治愈了。我现在得了一种不知 道什么病,我睁眼闭眼都是你,我现在病入膏肓,濒临死亡。你就是我的解药,我请求你做我的妻子!
1.表示各种“目的”,用for (1)What do you study English for 你为什么要学英语? (2)went to france for holiday. 她到法国度假去了。 (3)These books are written for pupils. 这些书是为学生些的。 (4)hope for the best, prepare for the worst. 作最好的打算,作最坏的准备。 2.“对于”用for (1)She has a liking for painting. 她爱好绘画。 (2)She had a natural gift for teaching. 她对教学有天赋/ 3.表示“赞成、同情”,用for (1)Are you for the idea or against it 你是支持还是反对这个想法? (2)He expresses sympathy for the common people.. 他表现了对普通老百姓的同情。 (3)I felt deeply sorry for my friend who was very ill. 4. 表示“因为,由于”(常有较活译法),用for (1)Thank you for coming. 谢谢你来。
(2)France is famous for its wines. 法国因酒而出名。 5.当事人对某事的主观看法,“对于(某人),对…来说”,(多和形容词连用),用介词to,不用for. (1)He said that money was not important to him. 他说钱对他并不重要。 (2)To her it was rather unusual. 对她来说这是相当不寻常的。 (3)They are cruel to animals. 他们对动物很残忍。 6.和fit, good, bad, useful, suitable 等形容词连用,表示“适宜,适合”,用for。(1)Some training will make them fit for the job. 经过一段训练,他们会胜任这项工作的。 (2)Exercises are good for health. 锻炼有益于健康。 (3)Smoking and drinking are bad for health. 抽烟喝酒对健康有害。 (4)You are not suited for the kind of work you are doing. 7. 表示不定式逻辑上的主语,可以用在主语、表语、状语、定语中。 (1)It would be best for you to write to him. (2) The simple thing is for him to resign at once.
常用介词用法(for-to-with-of)
For的用法 1. 表示“当作、作为”。如: I like some bread and milk for breakfast. 我喜欢把面包和牛奶作为早餐。 What will we have for supper? 我们晚餐吃什么? 2. 表示理由或原因,意为“因为、由于”。如: Thank you for helping me with my English. 谢谢你帮我学习英语。 3. 表示动作的对象或接受者,意为“给……”、“对…… (而言)”。如: Let me pick it up for you. 让我为你捡起来。Watching TV too much is bad for your health. 看电视太多有害于你的健康。 4. 表示时间、距离,意为“计、达”。如: I usually do the running for an hour in the morning. 我早晨通常跑步一小时。 We will stay there for two days. 我们将在那里逗留两天。
5. 表示去向、目的,意为“向、往、取、买”等。如: Let’s go for a walk. 我们出去散步吧。 I came here for my schoolbag.我来这儿取书包。 I paid twenty yuan for the dictionary. 我花了20元买这本词典。 6. 表示所属关系或用途,意为“为、适于……的”。如: It’s time for school. 到上学的时间了。 Here is a letter for you. 这儿有你的一封信。 7. 表示“支持、赞成”。如: Are you for this plan or against it? 你是支持还是反对这个计划? 8. 用于一些固定搭配中。如: Who are you waiting for? 你在等谁? For example, Mr Green is a kind teacher. 比如,格林先生是一位心地善良的老师。
加入收藏夹 主题: 介词试题It’s + 形容词 + of sb. to do sth.和It’s + 形容词 + for sb. to do sth.的用法区别。 内容: It's very nice___pictures for me. A.of you to draw B.for you to draw C.for you drawing C.of you drawing 提交人:杨天若时间:1/23/2008 20:5:54 主题:for 与of 的辨别 内容:It's very nice___pictures for me. A.of you to draw B.for you to draw C.for you drawing C.of you drawing 答:选A 解析:该题考查的句型It’s + 形容词+ of sb. to do sth.和It’s +形容词+ for sb. to do sth.的用法区别。 “It’s + 形容词+ to do sth.”中常用of或for引出不定式的行为者,究竟用of sb.还是用for sb.,取决于前面的形容词。 1) 若形容词是描述不定式行为者的性格、品质的,如kind,good,nice,right,wrong,clever,careless,polite,foolish等,用of sb. 例: It’s very kind of you to help me. 你能帮我,真好。 It’s clever of you to work out the maths problem. 你真聪明,解出了这道数学题。 2) 若形容词仅仅是描述事物,不是对不定式行为者的品格进行评价,用for sb.,这类形容词有difficult,easy,hard,important,dangerous,(im)possible等。例: It’s very dangerous for children to cross the busy street. 对孩子们来说,穿过繁忙的街道很危险。 It’s difficult for u s to finish the work. 对我们来说,完成这项工作很困难。 for 与of 的辨别方法: 用介词后面的代词作主语,用介词前边的形容词作表语,造个句子。如果道理上通顺用of,不通则用for. 如: You are nice.(通顺,所以应用of)。 He is hard.(人是困难的,不通,因此应用for.) 由此可知,该题的正确答案应该为A项。 提交人:f7_liyf 时间:1/24/2008 11:18:42
to和for的用法有什么不同(一) 一、引出间接宾语时的区别 两者都可以引出间接宾语,但要根据不同的动词分别选用介词to 或for,具体应注意以下三种情况: 1. 在give, pass, hand, lend, send, tell, bring, show, pay, read, return, write, offer, teach, throw 等之后接介词to。如: 请把那本字典递给我。 正:Please hand me that dictionary. 正:Please hand that dictionary to me. 她去年教我们的音乐。 正:She taught us music last year. 正:She taught music to us last year. 2. 在buy, make, get, order, cook, sing, fetch, play, find, paint, choose, prepare, spare 等之后用介词for 。如: 他为我们唱了首英语歌。 正:He sang us an English song. 正:He sang an English song for us. 请帮我把钥匙找到。 正:Please find me the keys. 正:Please find the keys for me. 能耽搁你几分钟吗(即你能为我抽出几分钟吗)? 正:Can you spare me a few minutes?
正:Can you spare a few minutes for me? 3. 有的动词由于用法和含义不同,用介词to 或for 都是可能的。如: do sb a favor=do a favor for sb 帮某人的忙 do sb harm=do harm to sb 对某人有害 在有的情况下,可能既不用for 也不用to,而用其他的介词。如: play sb a trick=play a trick on sb 作弄某人 请比较: play sb some folk songs=play some folk songs for sb 给某人演奏民歌 有时同一个动词,由于用法不同,所搭配的介词也可能不同,如leave sbsth 这一结构,若表示一般意义的为某人留下某物,则用介词for 引出间接宾语,即说leave sth for sb;若表示某人死后遗留下某物,则用介词to 引出间接宾语,即说leave sth to sb。如: Would you like to leave him a message? / Would you like to leave a message for him? 你要不要给他留个话? Her father left her a large fortune. / Her father left a large fortune to her. 她父亲死后给她留下了一大笔财产。 二、表示目标或方向的区别 两者均可表示目标、目的地、方向等,此时也要根据不同动词分别对待。如: 1. 在come, go, walk, move, fly, ride, drive, march, return 等动词之后通常用介词to 表示目标或目的地。如: He has gone to Shanghai. 他到上海去了。 They walked to a river. 他们走到一条河边。
keep的用法 1. 用作及物动词 ①意为"保存;保留;保持;保守"。如: Could you keep these letters for me, please? 你能替我保存这些信吗? ②意为"遵守;维护"。如: Everyone must keep the rules. 人人必须遵守规章制度。 The teacher is keeping order in class.老师正在课堂上维持秩序。 ③意为"使……保持某种(状态、位置或动作等)"。这时要在keep的宾语后接补足语,构 成复合宾语。其中宾语补足语通常由形容词、副词、介词短语、现在分词和过去分词等充当。如: 例:We should keep our classroom clean and tidy.(形容词) 我们应保持教室整洁干净。 You'd better keep the child away from the fire.(副词)你最好让孩子离火远一点。 The bad weather keeps us inside the house.(介词短语)坏天气使我们不能出门。 Don't keep me waiting for long.(现在分词)别让我等太久。 The other students in the class keep their eyes closed.(过去分词) 班上其他同学都闭着眼睛。 2. 用作连系动词 构成系表结构:keep+表语,意为"保持,继续(处于某种状态)"。其中表语可用形容词、副词、介词短语等充当。如: 例:You must look after yourself and keep healthy.(形容词) 你必须照顾好自己,保持身体健康。 Keep off the grass.(副词)请勿践踏草地。 Traffic in Britain keeps to the left.(介词短语)英国的交通是靠左边行驶的。 注意:一般情况下,keep后接形容词较为多见。再如: She knew she must keep calm.她知道她必须保持镇静。 Please keep silent in class.课堂上请保持安静。 3. ①keep doing sth. 意为"继续干某事",表示不间断地持续干某事,keep后不 能接不定式或表示静止状态的v-ing形式,而必须接延续性的动词。 例:He kept working all day, because he wanted to finish the work on time. 他整天都在不停地工作,因为他想准时完成工作。 Keep passing the ball to each other, and you'll be OK.坚持互相传球,你们就
202X中考英语:to和for的区别与用法中考栏目我为考生们整理了“202X中考英语:to和for的区别与用法”,希望能帮到大家,想了解更多考试资讯,本网站的及时更新哦。 202X中考英语:to和for的区别与用法 to和for的区别与用法是什么 一般情况下, to后面常接对象; for后面表示原因与目的为多。 Thank you for helping me. Thanks to all of you. to sb. 表示对某人有直接影响比如,食物对某人好或者不好就用to; for 表示从意义、价值等间接角度来说,例如对某人而言是重要的,就用for. for和to这两个介词,意义丰富,用法复杂。这里仅就它们主要用法进行比较。 1. 表示各种“目的” 1. What do you study English for? 你为什么要学英语? 2. She went to france for holiday. 她到法国度假去了。 3. These books are written for pupils. 这些书是为学生些的。 4. hope for the best, prepare for the worst. 作最好的打算,作最坏的准备。
2.对于 1.She has a liking for painting. 她爱好绘画。 2.She had a natural gift for teaching. 她对教学有天赋。 3.表示赞成同情,用for不用to. 1. Are you for the idea or against it? 你是支持还是反对这个想法? 2. He expresses sympathy for the common people.. 他表现了对普通老百姓的同情。 3. I felt deeply sorry for my friend who was very ill. 4 for表示因为,由于(常有较活译法) 1.Thank you for coming. 谢谢你来。 2. France is famous for its wines. 法国因酒而出名。 5.当事人对某事的主观看法,对于(某人),对?来说(多和形容词连用)用介词to,不用for.. He said that money was not important to him. 他说钱对他并不重要。 To her it was rather unusual. 对她来说这是相当不寻常的。 They are cruel to animals. 他们对动物很残忍。