第19卷第3期原子与分子物理学报Vol.19,(.3 )**)年+月,-./0102345/6738693:.,6/;:370,4765<-=1.,12>l.,)**)
?研究简讯?
Article ID:1000?0364(2002)03?0379?03
Site selective excitation spectroscopy of CsCdBr3:U3+*
=./:@A1,B54<62,2
(1.1CD>EC>DF5FGFHDEI7HJoDHC@oDK,4A@LFDG@CK oM1E@FAEF HAN9FEIAoloOK oM,I@AH,-FMF@230026;
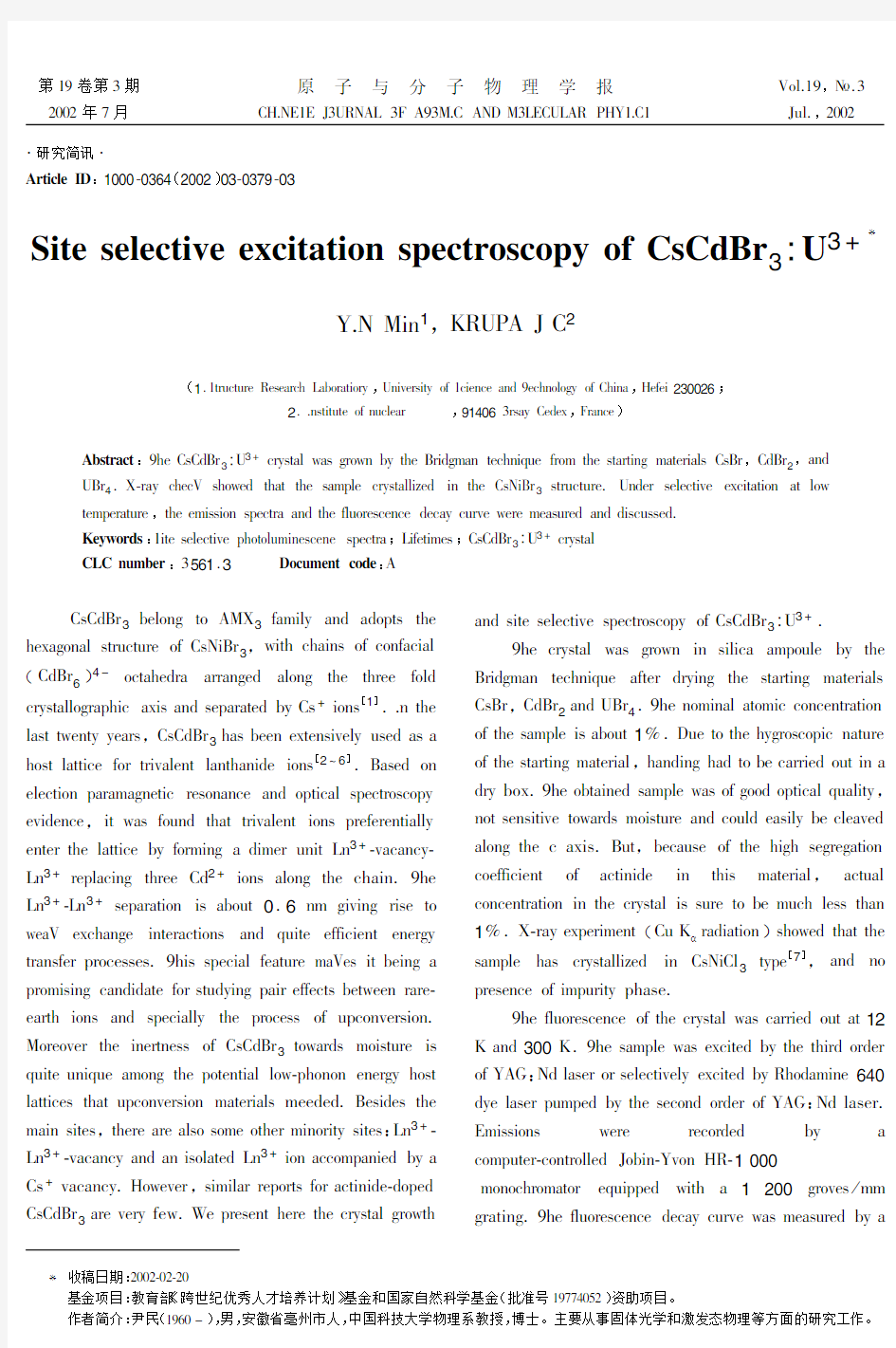
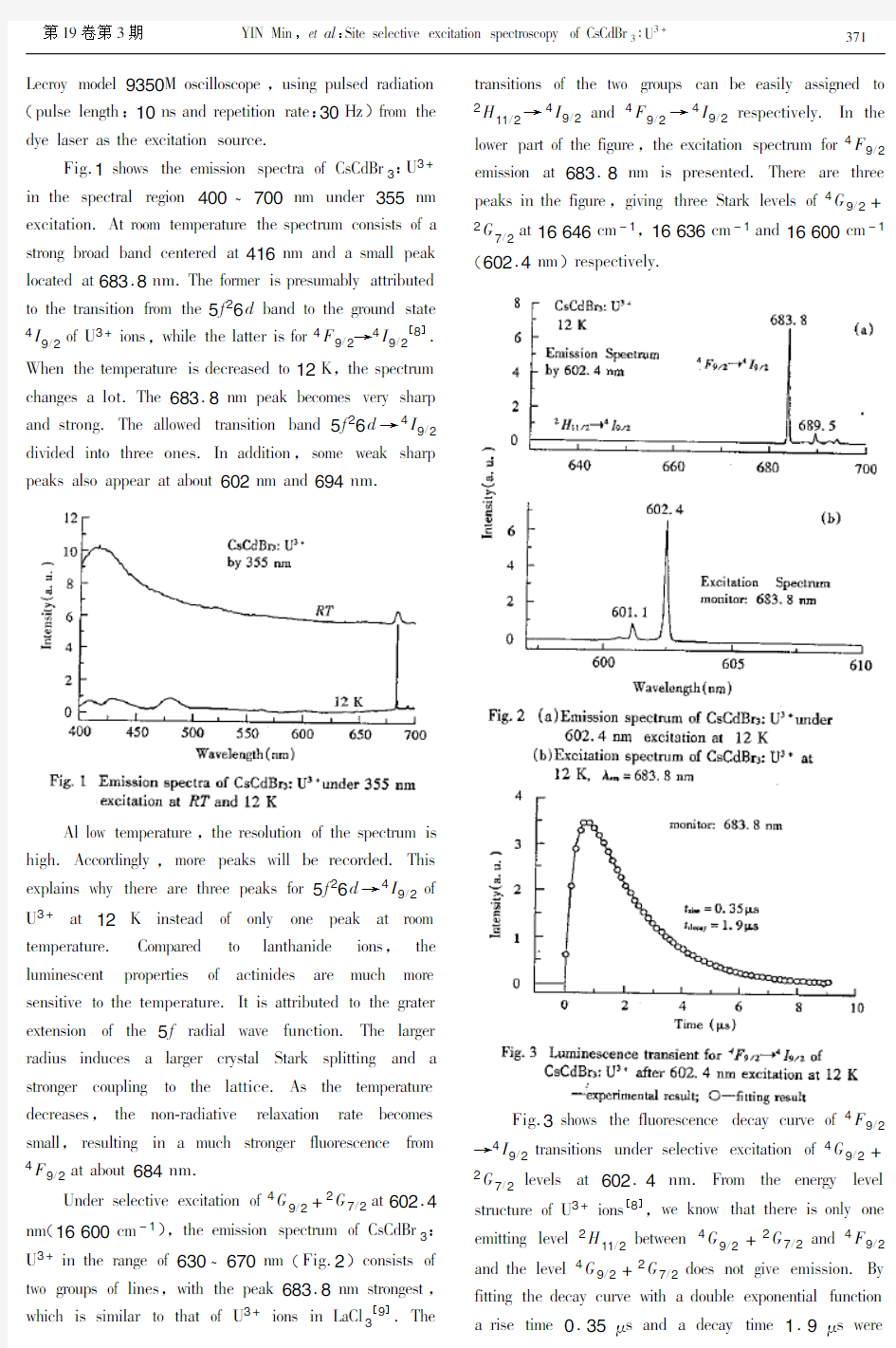
2..AGC@C>CF oM A>ElFHD Abstract:9IF,G,NQD3:43+EDKGCHl RHG ODoRA JK CIF QD@NOSHA CFEIA@T>F MDoS CIF GCHDC@AO SHCFD@HlG,GQD,,NQD2,HAN 4QD4.U?DHK EIFEV GIoRFN CIHC CIF GHSWlF EDKGCHll@XFN@A CIF,G/@QD3GCD>EC>DF.4ANFD GFlFEC@LF FPE@CHC@oA HC loR CFSWFDHC>DF,CIF FS@GG@oA GWFECDH HAN CIF Ml>oDFGEFAEF NFEHK E>DLF RFDF SFHG>DFN HAN N@GE>GGFN. Keywords:1@CF GFlFEC@LF WIoCol>S@AFGEFAF GWFECDH;7@MFC@SFG;,G,NQD3:43+EDKGCHl CLC number:3561.3Document code:6 ,G,NQD3JFloAO Co6:U3MHS@lK HAN HNoWCG CIF IFPHOoAHl GCD>EC>DF oM,G/@QD3,R@CI EIH@AG oM EoAMHE@Hl (,NQD 6 )4-oECHIFNDH HDDHAOFN HloAO CIF CIDFF MolN EDKGCHlloODHWI@E HP@G HAN GFWHDHCFN JK,G+@oAG[1]..A CIF lHGC CRFACK KFHDG,,G,NQD3IHG JFFA FPCFAG@LFlK>GFN HG H IoGC lHCC@EF MoD CD@LHlFAC lHACIHA@NF@oAG[2~6].QHGFN oA FlFEC@oA WHDHSHOAFC@E DFGoAHAEF HAN oWC@EHl GWFECDoGEoWK FL@NFAEF,@C RHG Mo>AN CIHC CD@LHlFAC@oAG WDFMFDFAC@HllK FACFD CIF lHCC@EF JK MoDS@AO H N@SFD>A@C7A3+?LHEHAEK? 7A3+DFWlHE@AO CIDFF,N2+@oAG HloAO CIF EIH@A.9IF 7A3+?7A3+GFWHDHC@oA@G HJo>C0.6AS O@L@AO D@GF Co RFHV FPEIHAOF@ACFDHEC@oAG HAN T>@CF FMM@E@FAC FAFDOK CDHAGMFD WDoEFGGFG.9I@G GWFE@Hl MFHC>DF SHVFG@C JF@AO H WDoS@G@AO EHAN@NHCF MoD GC>NK@AO WH@D FMMFECG JFCRFFA DHDF? FHDCI@oAG HAN GWFE@HllK CIF WDoEFGG oM>WEoALFDG@oA. :oDFoLFD CIF@AFDCAFGG oM,G,NQD3CoRHDNG So@GC>DF@G T>@CF>A@T>F HSoAO CIF WoCFAC@Hl loR?WIoAoA FAFDOK IoGC lHCC@EFG CIHC>WEoALFDG@oA SHCFD@HlG SFFNFN.QFG@NFG CIF SH@A G@CFG,CIFDF HDF HlGo GoSF oCIFD S@AoD@CK G@CFG:7A3+? 7A3+?LHEHAEK HAN HA@GolHCFN7A3+@oA HEEoSWHA@FN JK H ,G+LHEHAEK.-oRFLFD,G@S@lHD DFWoDCG MoD HEC@A@NF?NoWFN ,G,NQD3HDF LFDK MFR.YF WDFGFAC IFDF CIF EDKGCHl ODoRCI HAN G@CF GFlFEC@LF GWFECDoGEoWK oM,G,NQD3:43+. 9IF EDKGCHl RHG ODoRA@A G@l@EH HSWo>lF JK CIF QD@NOSHA CFEIA@T>F HMCFD NDK@AO CIF GCHDC@AO SHCFD@HlG ,GQD,,NQD2HAN4QD4.9IF AoS@AHl HCoS@E EoAEFACDHC@oA oM CIF GHSWlF@G HJo>C1Z.;>F Co CIF IKODoGEoW@E AHC>DF oM CIF GCHDC@AO SHCFD@Hl,IHAN@AO IHN Co JF EHDD@FN o>C@A H NDK JoP.9IF oJCH@AFN GHSWlF RHG oM OooN oWC@EHl T>Hl@CK,AoC GFAG@C@LF CoRHDNG So@GC>DF HAN Eo>lN FHG@lK JF ElFHLFN HloAO CIF E HP@G.Q>C,JFEH>GF oM CIF I@OI GFODFOHC@oA EoFMM@E@FAC oM HEC@A@NF@A CI@G SHCFD@Hl,HEC>Hl EoAEFACDHC@oA@A CIF EDKGCHl@G G>DF Co JF S>EI lFGG CIHA 1Z.U?DHK FPWFD@SFAC(,>B α DHN@HC@oA)GIoRFN CIHC CIF GHSWlF IHG EDKGCHll@XFN@A,G/@,l3CKWF[7],HAN Ao WDFGFAEF oM@SW>D@CK WIHGF. 9IF Ml>oDFGEFAEF oM CIF EDKGCHl RHG EHDD@FN o>C HC12 B HAN300B.9IF GHSWlF RHG FPE@CFN JK CIF CI@DN oDNFD oM=6[:/N lHGFD oD GFlFEC@LFlK FPE@CFN JK5IoNHS@AF640 NKF lHGFD W>SWFN JK CIF GFEoAN oDNFD oM=6[:/N lHGFD. 0S@GG@oAG RFDF DFEoDNFN JK H EoSW>CFD?EoACDollFN2oJ@A?=LoA-5?1000 SoAoEIDoSHCoD FT>@WWFN R@CI H1200ODoLFG\SS ODHC@AO.9IF Ml>oDFGEFAEF NFEHK E>DLF RHG SFHG>DFN JK H *收稿日期:)**)?*)?)* 基金项目:教育部《跨世纪优秀人才培养计划》基金和国家自然科学基金(批准号19++]*^))资助项目。 作者简介:尹民(19_*‘),男,安徽省亳州市人,中国科技大学物理系教授,博士。主要从事固体光学和激发态物理等方面的研究工作。 Lecroy model9350M oscilloscope,using pulsed radiation (pulse length:10ns and repetition rate:30Hz)from the dye laser as the excitation source. Fig.1shows the emission spectra of CsCdBr3:U3+ in the spectral region400~700nm under355nm excitation.At room temperature the spectrum consists of a strong broad band centered at416nm and a small peak located at683.8nm.The former is presumably attributed to the transition from the5f26d band to the ground state 4I 9/2 of U3+ions,while the latter is for4F9/2→4I9/2[8]. When the temperature is decreased to12K,the spectrum changes a lot.The683.8nm peak becomes very sharp and strong.The allowed transition band5f26d→4I9/2 divided into three ones.In addition,some weak sharp peaks also appear at about602nm and 694nm. Al low temperature,the resolution of the spectrum is high.Accordingly,more peaks will be recorded.This explains why there are three peaks for5f26d→4I9/2of U3+at12K instead of only one peak at room https://www.doczj.com/doc/763677608.html,pared to lanthanide ions,the luminescent properties of actinides are much more sensitive to the temperature.It is attributed to the grater extension of the5f radial wave function.The larger radius induces a larger crystal Stark splitting and a stronger coupling to the lattice.As the temperature decreases,the non-radiative relaxation rate becomes small,resulting in a much stronger fluorescence from 4F 9/2 at about684nm. Under selective excitation of4G9/2+2G7/2at602.4 nm(16600cm-1),the emission spectrum of CsCdBr3:U3+in the range of630~670nm(Fig.2)consists of two groups of lines,with the peak683.8nm strongest,which is similar to that of U3+ions in LaCl3[9].The transitions of the two groups can be easily assigned to 2H 11/2 →4I9/2and4F9/2→4I9/2respectively.In the lower part of the figure,the excitation spectrum for4F9/2 emission at683.8nm is presented.There are three peaks in the figure,giving three Stark levels of4G9/2+ 2G 7/2 at16646cm-1,16636cm-1and16600cm-1(602.4nm )respectively. Fig.3shows the fluorescence decay curve of4F9/2→4I9/2transitions under selective excitation of4G9/2+ 2G 7/2 levels at602.4nm.From the energy level structure of U3+ions[8],we know that there is only one emitting level2H11/2between4G9/2+2G7/2and4F9/2 and the level4G9/2+2G7/2does not give emission.By fitting the decay curve with a double exponential function a rise time0.35μs and a decay time1.9μs were 173 第19卷第3期YIN Min,et al:Site selective excitation spectroscopy of CsCdBr 3 :U3+ obtained.It is easy to know that the decay time1.9μs corresponds to the lifetime of4F9/2level and0.35μs is for2H 11/2 .They are much shorter than the same levels in LaCl3[10].The lifetime of an excited state is determined by both radiative and nonrediative depopulation processes.The latter depends on the energies of the accepting phonon models,and the so-called energy gap law can be used to express the multiphonon-relaxation rate constantω0=βe-αβ[11].Hereα,βare parameters characteristic of the material.p=ΔE/hωis the number of phonons of energy hωrequired to bridge the energy gap ΔE.The highest-energy phonons are the most efficient accepting https://www.doczj.com/doc/763677608.html,ually,multiphonon relaxation is dominant for the process of p<5.The highest phonon energy in chlorides is~260cm-1.For CsCdBr3a value of163cm-1was determined by Raman spectoscopy[12]. we can see that[8]in LaCl3,5phonons are needed to bridge the energy gap between4F9/2and its nearest4G7/2 level.While in CsCdBr3,about8phonons are required. But the measured lifetime of4F9/2level in CsCdBr3is much shorter than that in LaCl3.One reasonable reason for this is the formation of U3+-Cd vacancy-U3+pairs. The interaction and energy transfer between U3+ion in pairs pronouncedly decreases the lifetime of4F9/2level. In conclusion,new crystal CsCdBr3:U3+was grown by the Bridgman https://www.doczj.com/doc/763677608.html,pared to room temperature,the crystal presents a much stronger emission at low temperature.Under selective excitation at12K,fluorescence from2H11/2and4F9/2levels was recorded,with a quite intense emission at683.8nm.The lifetimes of2H11/2and4F9/2levels were obtained and discussed. REFERENCES [1]Neukum J,Bodenschatz N and Heber J.Spectroscopy and up- conversion of CsCdBr3:Pr3+[J].Phys.Rev.,1994,B50(6):3536~3546. [2]Antic-Fidancev E,Lemaitre-Blaise M,Chaminade J P and Porcher P.Crystal-field effect in CsCdBr3:Pr3+[J].J.Alloy and Compounds,1995,225(1~2):95~98. [3]Virey E,Couchaud M,Faure C,Ferrand B,Wyon C and Borel C.Room temperature fluorescence of CsCdBr3:Re(Re= Pr,Nd,Dy,Ho,Er,Tm)in the3~5μm range[J].J. Alloys and Compounds,1998,275~277:311~314. [4]Wermuth M,Riedener T and Gudel H U.Spectroscopy and upconversion mechanisms of CsCdBr3:Dy3+[J].Phys.Rev.,1998,B57(8):4369~4376. [5]Bodenschatz N,Neukum J and Heber J.Quantum up-conversion of Tm3+and Tm3+-Ho3+ion pairs in CsCdBr3[J]. J.Lumin.,1995,66~7(1~6):213~218. [6]Goldner P and Pelle F.Site selection and up-conversion studies in erbium and ytterbium doped CsCdBr3[J].J.Lumin.,1993,55(4):197~207. [7]JCPDS file No.24~0237. [8]Crosswhite H M,Crosswhite H,Carnall W T and Paszek A P. Spectrum analysis of U3+:LaCl3[J].J.Chem.Phys.,1980,72(9):5103~5117. [9]Yin M,Joubert M F and Krupa J C.Green upconversion and infrared emission spectra of U3+in LaCl3[J].J.Alloys and Compounds,1997,260:64~69. [10]Yin M,Joubert M F and Krupa J C.Infrared to green up-conversion in LaCl3:U3+[J].J.Lumin.,1997,75:221~ 227. [11]Riseberg L A and Moos H W.Multiphonon orbit-lattice relexation of excited state of rare-earth ions in crystals[J]. Phys.Rev.,1968,174(2):429~438. [12]Pilla O,Cazzanelli E,Blanzat B,Andraud C and Pelle F. Comparative Raman study of phonon linewidths in pure and lead-doped CsCdBr3[J].Phys.Status.Solidi,1987,B144 (2):845~851. 273原子与分子物理学报2002年