
****************** Exact Seamless Steel Tube Co.,Ltd.
Quality Management System Document
Quality Manaul
No.:SY-ZS-2014
Edition:A
Revised Status:2
Controlled No.:02
Controlled Status:Controlled
Write:Quality Management Dept.
Review:
Approval:
Issued Date:2014.4.10 Valid Date:2014.4.20 *****************Exact Seamless Steel Tube Co.,Ltd.Issued
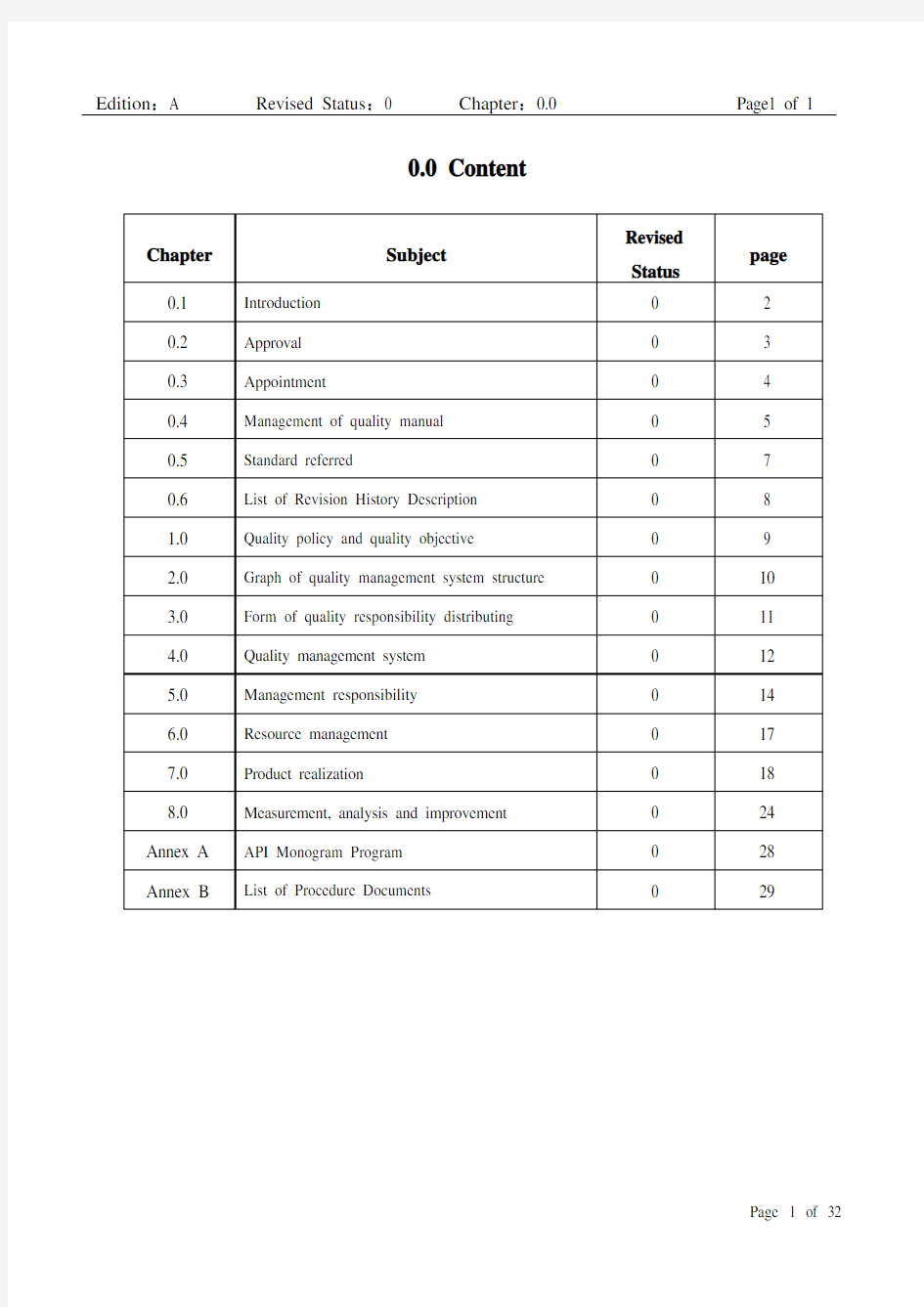
0.0 Content
0.1 Introduction
************** Exact Seamless Steel Tube Co.,Ltd. was established in 1997, which is specializing in producing tubing plain end,cold-drawing seamless steel tube, cold-rolled accurate seamless steel tube, the slippery post shock absorber. There are 360 employees including more than 60 technicians.
The company obtained the certificate of ISO 9001 in the year of 2004.The company is the qualified suppliers to our customer and so on. We won good prestige by good quality and duly delivery.
The company will try best to provide the good service to all new and old customers based on the advanced technology, high quality product, reasonable price, we sincerely expect the chance to cooperate with you, and create wonderul future.
General Manager:
Post Code:
Add:
Tel:
Fax:
0.2 A pproval
In order to suit the market, ensure the products meet the requirement of customer, the company adopted process approach when developing, implementing and improving the effectiveness of the quality management system. The company established the Quality Manual, edition A, based on the standards of API Spec Q1-2003,the 7th edition, Specification for Quality Program for the Petroleum Petrochemical and Natural Gas Industry, and GB/T 1 9001-2008 idt IS09001:2008, Quality Management System—Requirements as well as the real situation of the company, the Quality Manual is now approved to be issued and put into effect from Jan.
20.2006.
The manual specifies our company’s quality policy and objectives, outlines our quality system and describes the requirements and basic rule of its implementation, and identifies the procedure documents for main processes. The manual affords our active guideline of action. All employees of the company must strictly comply with the specific requirements of the manual.
General Manager:
Apr. 8,2014
0.3 A ppointment
In order to carry out IS09001:2008 and API Spec Q1:2003(7th edition), strengthen the leadership on quality management system operation, the General Manager appoints the Mr. Yuan Xia Ping are as the management representative, the responsibilities and authorities are:
a) Ensuring that processes needed for the quality management system are established, implemented and maintained.
b) To report the General Manager the situation of the quality system movement, hold liaison with external organizations, conduct audits, adjustment, coordination and reviews inside this group.
c) Ensuring the promotion of awareness of customer requirements throughout the company.
d) Responsible for the external relations relative to the quality management system.
General Manager:
Oct. 8.2014
0.4 M anagement of quality manual
1 Contents of the Quailty Manual
The qualtiy manaul was established based on the standards of API Spec Q1:2003, the specification for qulatiy programe for the petroleum, petrochemical and natural gas industry, and GB/T 19001—2008 idt IS09001:2008, quality management system-requirements as well as the real situation of the company, which includes:
a)The scope of the quality management system relating to the raw material purchasing, design, manaufacture, inspection, sales and service of square drill stem globe vlave,drill stem joints and switchover joints, there is no any exclusions to the standards.
b) The documented procedures established for the quality management system.
c) The description of the interaction between the processes of the quality management system.
2 Terms and Definitions
The terms and definitions given in GB/T 19000—2008 idt ISO 9000:2008, quality management system-fundamentals and vocabulary and section 3 of API Spec Q1:2003 shall apply.
3 Control of Outsourcing Process
Control and monitor the outsource processes (such as hydrostatic tests) according to the procedure Control Procedure of Purchasing.
4 Management of the Quality manual
4.1 The Quality Manual shall be approved by the General Manager prior to its issue. The Quality Management Dept. is responsible for the management of the Quality manual, no one can provide the quality manual to the person outside of the company without the approval of the management representative. When the manual holder leaves the company, the quality manaul shall be returned to The Quality Management Dept. and relative procedures shall be gone through.
4.2The manual holder shall properly keep the manual, any loss, damage and arbitrarily alteration shall be prohibited.
5 Changes to the quality manual
5.1 The need of changing quality manual shall be reviewed by the management representaive, approved by the general manager pior to its implementation.
5.2 The Quality Manual was combined by every independent chapter,there was identification of revised status and page No.for every chapter.Therefore,each chapter can be revised solely,which would not affect the edition of the quality manaul.The Arabic numbers0,1,2,3….will be used to identify the resivised status.
5.3 After changing the Quality Manual, The Quality Management Dept. shall revise the relative controlled documents accordingly, reclaim the old chapter(page)and dispose them according to the requirements of Procedure of Document Contro1.
5.4 The uncontrolled copy of Quality ManuaI can be provided to the outside of the company after the approval of the General Manager or Management Representative,The Quality Management Dept. shall make relative record. but will not foIlow its validity.
5.5 Normally, the reviewed on the quality manaul will be conducted once every year according to the requirement of Procedure of Document Control; the frequency can be increased as necessary, if any changes needed to the Qualtiy ManauI after the review, the item 5.1,5.2 and 5.3 shall be implemented.
6 Change to the edition of Quality Manual
6.1 The change to the quality manual shall be approved by the general manager.
6.2 The edition of the quality manual can be changed for the one or more of following situations:
a) Big change to product;
b) Big change to the applicable scope of quality manual;
c) Edition change of standard referred;
d) Big change to company’s organization structure.
6.3The No. of edition shall be identified by the letter A,B,C….
6.4 The Quality Management Dept. shall duly release the controlled copy of the new edition quality manual and reclaim the old edition, the procedure of document control shall be implemented accordingly.
0.5 S tandard Referred
1, API Spec Q1:2003 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry.
2, GB/T 19001—2008 idt ISO 9001:2008 Quality Management System-Requirements.
3, API Spec 5CT-2005 Casing and Tubing
1.0 Quality policy and quality objective
Quality Policy
Scientence Management
Base on Quality
Continuous Improment
Customer Satisfaction
Quality Objectives
a) The first inspection rate is over 97%;
b) The customer satisfaction is over 96%.
General Manager:
Dec. 8,2014
2.0 Graph of Quality Management System Structure
Remark: ☆Directed Leading ▲Main Responsibility △Assortment Management
4.0 Quality Management System
1 Purpose
Establish, document, implement and maintain a quality management system and continually improve its effectiveness in accordance with the requirements of IS09001:2008&API Spec Q1(7th)standard.
2 Scope
It is applicable to the control of the quality management system and document system.
3 Requirements
3.1 General requirements of quality system
3.1.1 All requirements without exclusion of API Spec Q1-2003 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry and ISO 9001:2008(GB/T 19001-2008)Quality Management System-Requirements are applied for the quality management system of the company. The company establish, document, implement and maintain a quality management system and continually improve its effectiveness in accordance with the requirements of API Spec Q1-2003 and GB/T19001-2008 idt ISO9001:2008.
3.1.2 The Management Representative shall organize every Department to plan the process of quality management system.
a) Conforms to the actual status of our company and requirement of the standard, the processes needed by the
quality management system are divided into 25 linking up each other and clear responsibility processes, and generates relative documented procedure,
b) Determine criteria and methods needed to ensure that both the operation and control of these processes are
effective (See relevant chapter of this manual and the procedures and records referenced.),
c) Ensure the availability of resources and information necessary to support the operation and monitoring
these processes,
d) Monitor, measure and analyse these processes,
e) Implement actions necessary to achieve planned results and continual improvement of these processes,
f) Control the outsource processes according to the procedure 《Control Procedure of Purchasing》.
3.1.3 These processes shall be managed by the company in accordance with the requirements of this standard.
3.2 Requirements of document
3.2.1 Quality management system documents
In according to dimensions, type, process complicated degree, and personnel ability to select and establish following type document. It include:
a) Documented statements of a quality policy and quality objectives;
b) A quality manual; The quality manual include:
i.Scope of quality management system,
ii.Quality policy and quality objectives,
https://www.doczj.com/doc/c95616459.html,anizational Structure and personnel responsibility,
iv.The documented procedures established for the quality management system, or reference to them,
v.Description of the interaction between the processes of the quality management system.(refer to Appendix C and Appendix D)
c) Procedure documents; include control of document, control of quality records, internal audit. Control of non-conformance product, Corrective and preventative action, Management review etc. procedure documents;
d) Documents needed by the company to ensure the effective planning, operation and control of its processes. And
e) Quality records.
The contents include: Scope of quality management system; procedure document shall be established; statement correlation of quality management system.
3.2.2 Control of documents
Our company establish and maintain 《Control of Document Procedure》in order to control all documents and data that relates to the requirements of API and the other relevant product specifications including to the extent of the applicable documents of external origin such as standards and customer drawings.
a)To be approved by authorized personnel to ensure documents for adequacy prior to issue;
b)To review and update periodically and non-periodically and re-approve documents;
c)To ensure that changes are identified;
d)To ensure that relevant versions of applicable documents are available at points of use;
e)To ensure that documents remain legible and readily identifiable;
f)To ensure that documents of external origin (including standard) are identified and their distribution
controlled;
g)To prevent the unintended use of obsolete documents, and to apply suitable identification to them if they
are retained for any purpose;
h) A list identifying the current revision and up to date edition status of documents shall be established ;
i)The documents shall be changed by the original department (Any proposed change to the company’s
quality program, to a degree requiring changes to the quality manual, shall be submitted to API for
a cceptance prior to incorporation into the company’s quality program).
3.2.3 Control of records
Our company establish and maintain 《Control of Quality Records Procedure》to control quality records and provide documentary records in order to show that product’s quality meets specified requirements and to demonstrate achievement of the effective operation or implementation of the quality system.
a) The Quality Management Dept. shall establish and maintain documented procedures for collection, identification, access, filing, storage, maintenance and disposition of quality records.
b) The quality records with API monogrammed products or with other standard product shall meet API specification or other standard of product.
c) Related departments shall maintain the quality records, including the quality records of subcontractors, to demonstrate achievement of the effective operation or implementation of the quality system.
d) A quality records shall be legible and identifiable which shall distinguish any products quality records shall be stored in a suitable environment In order to prevent deterioration or loss. Quality records shall be stored and maintained in such a way that they are readily retrievable within facilities.
e) The Quality Management Dept. shall establish and document the retention period of quality records:
1) Records required by API product specification or other product specification shall be retained for period of time specified therein.
2) Records specific to demonstrate achievement of the effective operation of the quality system shall be maintained for a minimum of 5 years.
5.0 M anagement Responsibility
1 Purpose
Identifying the responsibility for the managers at all level in the management of quality management system, so as to ensure the establishment, maintenance and validity of the quality management system.
2 Scope
Applicable for the content stipulation for Management Commitment, Customer Focus, Quality Policy, Quality Objective, Quality Plan, Responsibility and Authority, Management Representative, Internal Communication, Management Review.
3 Requirements
3.1 Management commitment
The General Manager shall provide evidence of its commitment to the development and implementation of the quality management system and continually improving its effectiveness by communicating to the company the importance of meeting customer as well as statutory and regulatory requirements,
a) Should acquire the quality ideology, clearly knows the essential requirements of customer.
b) Establishing and approval the quality policy and objectives.
c) Conducting management review at planned intervals. See《Control of Management Review Procedure》.
d) To ensure that obtain necessary resource of quality management system running.
3.2 Customer focus
Company’s success depends on understanding and meet ing customer’s current and intending needs, and exceeding those requirements and expectation. General Manager and Supplying and Marketing Department should take customer’s satisfactory realization as objectives, therefore, he should:
Determine the customer’s needs and expectations. Convert customer’s needs and expectation to requirements. Satisfy the converted requirements.
3.3 Quality Policy
General Manager shall ensure that Quality Policy:
a) The quality policy is appropriate to the purpose of the company.
b) Show a commitment to comply with requirements and continually improve the effectiveness of the quality management system.
c) This quality policy provides a framework for establishing and reviewing quality objectives.
d) Ensure that this policy is understood, implemented and maintained at all levels of the company.
e) Quality Policy reviewed for continuing suitability and continual improvement.
3.4 Quality Objective
3.4.1 The Quality Management Dept. will propose the Quality Objective for the next year by the end of every year according to the objective achievement situation of the current year, the proposed Quality Objective will be implemented after the review and approval by Management Representative and highest manager; the Quality Objective of each department will be established by each department and submitted to Management Representative and highest manager for approval, and organized to implement by Department Manager. 《Control of Quality Objectives Procedure》shall be carried out.
3.4.2 The company establishes the quality objective for the relative departments on the basis of the analysis of the Quality Policy, the Objectives should be measurable, and consistent with the quality policy.
3.4.3 Quality Objective meets the requirements of the product.
3.5 Planning of quality management system
3.5.1 The highest manager is responsible for the planning with the assistance of Management Representative, the process, input, output, and resource needed identification shall be defined.
3.5.2 Planning Time:
a) When adopt new management standard or any change on the standard;
b) Major change on administration organization;
C) Major change on the resource preparation or market status;
d) Any issue not covered by the system.
3.5.3The quality management system planning shall ensure that:
a) The planning of the quality management system is carried out in order to meet the requirements of the item
4.1 of standard.
b) The integrity of the quality management system is maintained when changes to the quality management system are planned and implemented.
3.5.4 The quality management system planning shall carry out《Control of Quality Management System Planning Procedure》.
3.6 Responsibility and authority
To ensure the effective implementation of the quality system, General Manager establishes quality assurance system organization structure(see 2.0), applicable to the quality system and assigns the quality responsibility specified in 4 to 8 to responsible persons and defines the responsibility(see 3.0), especially those requiring independent execution of responsibility and authority, to provide an organizational foundation for functional positions to execute their responsibility and authority independently, so as to:
a) Initiate action to prevent the occurrence of product nonconformity;
b) Identify and record any product quality problems;
c) Initiate, recommend, or provide solutions through designated channel;
d) Verify the implementation of solutions;
e) Control further processing, delivery or installation of nonconforming products until the deficiency or unsatisfactory conditions has been corrected.
3.6.1General Manager shall have defined responsibility and authority for:
a)The implementation of national law, regulation and relevant police.
b)The establishment and approval of quality police, quality objective and ensuring the employees to understand
it.
c)Ensuring resources demand and sufficient resources provision, and determination of organization and
function responsibility.
d)In charge of management review and the continual effectiveness of Qality Management System.
e)In charge of product quality.
3.6.2 Management Representative shall have defined responsibility and authority. (see 3.7)
3.6.3 O ther department and personnel’s responsibility and authority
The company establish and maintain the procedure for control of responsibility and authority(See 《Responsibility, Authority and Communication》, and implement it.
3.7 Management Representative
To ensure the implementation of quality system requirement, General Manager appoints Management Representative who shall have been defined authorities, o ensure that a quality system is established and maintained in accordance with ISO 9001:2008 International Standard and API Spec Q1:2003:
a)Ensuring that processes needed for the quality management system are established, implemented
and maintained.
b)Reporting to General Manager on the performance of the quality management system and any need
for improvement..
c)Ensuring the promotion of awareness of customer requirements throughout the company.
d)Liaising with external parties on matters relating to the quality management system.
3.8 Internal Communication
The communication can be realized by team briefing, all kinds of meetings, notice board, internal publication and all kinds of medium to ensure the communication on the process of quality management system. Detail communication methods shall be described in every procedure documents.
3.9 Management review
3.9.1 In according to the requirement of《Control of Management Review Procedure》. General Manager reviews the quality system annually to ensure its continuous suitability and effectiveness in satisfying the requirements of API Specification and IS09001 standard (The spacing time of the twice management review is not more than 12 month). And temporary review is required when changes in the company’s internal or external environments, restructuring of products,
3.9.2 Management review is led by General Manager and participated by Management Representative, and responsible personnel in each department and the persons assigned by General Manager. The Quality Management Dept. is responsible for providing background information for management review.
3.9.3The management review includes,
a) Results of audits.
b) Customer feedback.
c) Process performance and product conformity.(Nonconformance)
d) Status of preventative and corrective action.
e) Follow-up actions from previous management reviews.
f) Changes that could affect the quality management system.
g) Recommendations for improvement.
h) Changes in API Spec. Requirements.
3.9.4 Management review output includes.
a) Improvement of the effectiveness of the quality management system and its processes.
b) Improvement of product related to customer requirements.
c) Resource needs.
3.9.5 Results of management review shall be record. The Quality Management Dept. is responsible for maintaining the records of management review, and preparing the report of management review (including corrective action). Those documents shall be reviewed by Management Representative and submitted to General Manager for approval before implementation.
6.0 Resource Management
1 Purpose
In order to timely provide the resource needed for customer satisfaction, and the establishment, maintenance and improvement of the quality management system.
2 Scope
Personnel, infrastructure, and working environment related to the quality system.
3 Requirements
3.1 Provision of resource
The company provides the resource for the establishment, maintenance and continual improvement of the quality management system; and the resource for customer satisfaction.
The General Manager is responsible for the resource provision, including personnel, funds and material.
3.2 Human resource
3.2.1 The Quality Management Dept. evaluate personnel who are assigned responsibilities defined in the quality management system shall be competent on the basis of applicable education, training, skills and experience.
3.2.2.Training, Awareness and Competence
The company establish and maintain the《Control of Human Resources Procedure》, The Quality Management Dept. implements the procedure to ensure:
a)Determine the necessary competence for personnel performing work affecting product quality.
b)Evaluate the necessary competence for personnel performing work affecting product quality and identify training need.
c)The training requirements shall provide for quality management system training and for job training of personnel.
d)The frequency of training shall be defined.
e)Provide on-the-job training for personnel in any new or modified job affecting product quality, including contract or agency personnel.
f)Provide training or take other actions to satisfy these needs.
g)Evaluate the effectiveness of the actions taken.
h)Ensure that its personnel are aware of the relevance and importance of their activities an dhow they contribute to the achievement of the quality objectives, and be informed about the consequences to the customer of nonconformity to quality requirements.
i)Maintain appropriate records of education, training, skills and experience.
The Quality Management Dept. establish and maintain the annual training plan and examine the personnel who perform the quality management, test, verification and special assigned tasks shall be qualified on the basis of appropriate education, training and experience. Only those who are accredited through examination can work at the posts. These personnel include: Inspection and test personnel; Internal quality audits personnel; Personnel performing special processes.
Training and qualification records shall be maintained by Quality Management Dept.
3.3 Infrastructure
The company determines and meets the needs required by the new product on working environment, equipment, hardware, software and supposing service,
The Production and Equipment Dept. is responsible to control of equipment according to 《Control of Infrastructure and Work Environment Procedure》.
3.4 Working Environment
To identify the requirement of working environment based on production technics, inspection method and equipment instruction, and meet the requirement by the item 3.3.
7.0 Product Realization
1 Purpose
In order to effectively control and manage the product realization processes.
2 Scope
The product realization processes related to the quality management system.
3 Requirements
3.1 Product realization planning
3.1.1 The company plans and develops the process needed for the product realization according to 《Control of Realization Processes Planning Procedure》, the Technical and Inspection Dept. is responsible for the implementation of the procedure.
3.1.2 Product realization process programming includes:
a) Determination of the quality objective and requirement of the product:
b) Working-out the specific process, document and resource provision for the product;
c) Determine product acceptance criterion and the validation, confirmation, supervision, inspection and testing activities needed by the product;
d) Record should be maintained.
3.2 Customer-related processes
3.2.1 The Supplying and Marketing Dept. is responsible to distinguish the needs and expectation of customer, confirm the requirements stipulated in the order:
a) Product requirements specified by customer, including the requirements for delivery and requirements after delivery;
b) Potential requirements not specified by customer;
c) The obligation related to the product, including the requirements of the law;
d) Any additional requirements confirmed by the company.
3.2.2 The procedure document 《Customer-related Processes Control Procedure》shall be established. The contract shall be review the requirements related to the product and any additional requirements determined by the company. It shall ensure that:
a) All requirements are adequately defined within the scopes of API specified standard and related product specifications and are documented.
b) Any differences between the contract or order requirements and those in the tender are resolved;
c) Our company has the capability to meet the contract or order requirements, and the requirements of API Spec.5CT or related product standard.
d) Where no written statement of requirement is available for an order received by verbal means, the company shall ensure that the order requirements are agreed before their acceptance.
3.2.3 Revisions to contract
The Supplying and Marketing Dept.shall revise contract in accordance with the procedure of contract review. The contract revisions shall be informed to all concerned departments.
3.2.4 The Supplying and Marketing Dept.shall maintain all records of contract review.
3.2.5 Customer Communication
The customer communication will be carried out according to 《Control of Production and Service Provision Procedure》, which includes:
a) Product information;
b) Enquiry, disposal of contract or tender including relative modification;
c) Customer feedback including customer complaint.
3.3 Design and development
The company established and implemented《Design and Development Control Procedure》, ensure the effective control on the product design, so as to meet the stipulated requirement.
3.3.1 Design and development planning
a) The Technical and Inspection Dept.shall make in accordance with the contract requirements and design duty. The plan shall describe or reference these activities and define responsibility for their implementation. Plans describe or reference development activity. The plans shall be updated, as the design evolves.
b) Design and design verification activities planned and assigned. The design and development activities shall be assigned to qualified personnel equipped with adequate resources to ensure that the specified requirements are met.
c) Documentation includes methods, assumptions, formulations and calculation.
d) Organizational and technical interfaces between different groups which input into the design process shall be defined and necessary information documented, transmitted and regularly reviewed.
e)Planning output shall be updated, as appropriate, as the design and development progresses.
3.3.2 Design and development inputs
a) Functional and performance requirements;
b) Applicable statutory and regulatory requirements;
c) Where applicable, information derived from previous similar designs, and
d) Incomplete, ambiguous or conflicting requirements shall be resolved with those responsible for imposing these requirements;
e) Design input shall take into consideration the results of any contract review activities;.
f) Input includes customer specified requirements.
3.3.3 Design and development outputs.
The outputs of design and development shall be provided in a form that enables verification against the design and development input, it shall includes:
a) Meet the input requirements for design and development;
b) Provide appropriate information for purchasing, production and for service provision;
c) Contain or reference product acceptance criteria, and
d) Specify the characteristics of the product that are essential for its safe and proper use.
Design and developments output documents shall be accepted prior to release.
3.3.4Design and development review
a) At appropriate stages during the design, the design results shall be reviewed;
b) Design review conducted by unoriginal design personnel and documented;
c) Final design review shall be conducted and documented by individuals other than the person or persons who developed the design;
d) The results of design review shall be documented and maintained.
3.3.5 Design and development verification
The professional technicians verify the design and development after it finished. Design and development verification shall be made through checking calculation, review of design output documents independent of activities of 7.3.4, comparing new designs to similar proven designs, and testing verification to ensure that the design
ABC Farms ISO 14001 - Environmental Management System Manual Prepared By: Approved By: Signature: ____________________________ Date: _________ / Revision #: 0 / Revision Date: / Effective Date:
SECTION 0 - TABLE OF CONTENTS MANUAL ISO SECTION SECTION TITLE PAGE SECTION 0TABLE OF CONTENTS x SECTION I INTRODUCTION x SECTION II DISTRIBUTION, REVISION & CONTROL x 4.2 4.2ENVIRONMENTAL POLICY x 4.3 4.3PLANNING x 4.3.1 4.3.1 Environmental Aspects x 4.3.2 4.3.2 Legal and Other Requirements x 4.3.3 4.3.3 Objectives and Targets x 4.3.4 4.3.4 Environmental Management Programs x 4.4 4.4IMPLEMENTATION & OPERATION x 4.4.1 4.4.1 Structure and Responsibility x 4.4.2 4.4.2 Training, Awareness and Competence x 4.4.3 4.4.3 Communication x 4.4.4 4.4.4 Environmental Management System Documentation x 4.4.5 4.4.5 Document Control x 4.4.6 4.4.6 Operational Control x 4.4.7 4.4.7 Emergency Preparedness and Response x 4.5 4.5CHECKING AND CORRECTIVE ACTION x 4.5.1 4.5.1 Monitoring and Measurement x 4.5.2 4.5.2 Non-conformance and Corrective Action x 4.5.3 4.5.3 Records Management x 4.5.4 4.5.4 Environmental Management System Audits x 4.6 4.6MANAGEMENT REVIEW x
ID 号:33526871 受控文件 编制: 汪斌,叶雁飞;文控审核: 赵建岭;会签: 何伟,丁云长,崔载福,陈卫德,胡雄旺,徐华2,徐海波;意见汇总: 汪斌,赵建岭;审核: 徐华2;批准: 刘向阳;发布目标: 赵建岭 归档日期:2012-04-20 17:16:38 编码: GL 质量管理体系文件 文件名称 吉利供应商质量手册 版 本 1 页码 / 文件编号 GLW000864 生效日期 2012年4月23日
供应商质量手册 (第一版) 浙江吉利控股集团有限公司
前 言 《供应商质量手册》是参考吉利公司的质量管理手册、程序文件,并结合当前吉利供应商管理的现状而编制的,以指导供应商在准入、零部件开发、量产初期及批量生产管理等过程中的需要开展哪些工作及提交哪些文件。 当前,吉利在不断提升自身竞争能力的同时,努力打造具有“三高一低”(高质量、高技术、高忠诚度和低成本)的、一流竞争力的供应商体系。相信通过相互合作、沟通理解和及时采取措施将有利于吉利和供应商双方事业的发展,最终实现“让世界充满吉利”的美好愿景! 本手册版权属于浙江吉利控股集团有限公司及其各子公司。 未经吉利的书面许可,本手册的任何内容都不能以任何形式和手段进行复制、储存和传送。供应商不得以任何方式将本手册中提到的吉利品质体系内容提供给第三者。 本手册将在运用过程中不断更新完善,各供应商朋友如在应用过程中发现不足,请及时反馈指正。 如有任何问题,请与相关SQE联系。 吉利集团质量管理部 供应商管理室 2012年4月
目录 第一章 供应商选点 (4) 第二章 先期产品质量策划 (6) 第一节 第一阶段供应商品熟评价 (6) 第二节 第二阶段供应商品熟评价 (25) 第三节 第三阶段供应商品熟评价 (34) 第四节 第四阶段供应商品熟评价 (53) 第五节 第五阶段供应商品熟评价 (59) 第六节 生产件批准程序(PPAP) (61) 第三章 量产管理 (66) 第一节 驻厂检验 (66) 第二节 受控发运 (69) 第三节 统计技术 (71) 第四节 质量改进 (73) 第五节 供应商审核管理 (80) 第六节 免检管理 (81) 第七节 供应商优化管理 (82) 第八节 供应商违约管理 (83) 第四章 供应商品质五星级评价 (84)
Ningbo XXX Material Technology Co.,Ltd ISO9001:2015 Quality Manual Revision [A/0] - [2018/3/1] (c) [Copyright Year Of 2018] [Ningbo XXX Material Technology Co.,Ltd]; all rights reserved. This document may contain proprietary information and may only be released to third parties with approval of management. Document is uncontrolled unless otherwise marked; uncontrolled documents are not subject to update notification.
Revision [A/0] - [2018/3/1] Page 1 of 19 TABLE OF CONTENTS 0.0 Revision History and Approval ...................................................................................................................... 3 1.0 Welcome to Ningbo XXX Material Technology Co.,Ltd ................................................................................ 4 2.0 XXX Material: Who We Are ........................................................................................................................... 4 2.1 Determining Our Strategic Direction ......................................................................................................... 4 2.2 Scope of the Management System ........................................................................................................... 4 2.2.1 Scope Statement ............................................................................................................................... 4 2.2.2 Facilities Within the Scope ................................................................................................................ 4 2.2.3 Permissible Exclusions ..................................................................................................................... 5 2.2.4 Scope of the ISO9001:2015 Quality Manual ..................................................................................... 5 3.0 Quality Policy................................................................................................................................................. 5 4.0 Management System Structure and Controls ............................................................................................... 5 4.1 Process Approach .................................................................................................................................... 5 4.1.1 Process Identification ........................................................................................................................ 5 4.1.2 Process Controls & Objectives .......................................................................................................... 6 4.1.3 Outsourced Processes ...................................................................................................................... 7 4.2 Documentation & Records ....................................................................................................................... 7 4.2.1 General .............................................................................................................................................. 7 4.2.2 Control of Documents ....................................................................................................................... 7 4.2.3 Control of Records ............................................................................................................................ 7 4.3 Change Management ................................................................................................................................ 8 4.4 Risks and Opportunities ............................................................................................................................ 8 5.0 Management & Leadership ........................................................................................................................... 8 5.1 Management Leadership and Commitment .............................................................................................. 8 5.2 Customer Focus ........................................................................................................................................ 9 5.3 Quality Policy ............................................................................................................................................. 9 5.4 Organizational Roles Responsibilities & Authorities ................................................................................. 9 5.5 Internal Communication ............................................................................................................................ 9 5.6 Management Review .............................................................................................................................. 10 6.0 Resources ................................................................................................................................................... 10 6.1 Provision of Resources ........................................................................................................................... 10 6.2 Human Resources .................................................................................................................................. 10 6.3 Infrastructure ........................................................................................................................................... 11 6.4 Work Environment ................................................................................................................................... 11 6.5 Organizational Knowledge ...................................................................................................................... 11 7.0 Operation ..................................................................................................................................................... 11 7.1 Operational Planning and Control ........................................................................................................... 12 7.2 Customer-Related Activities .................................................................................................................... 12 7.2.1 Capture of Customer Requirements ............................................................................................... 12 7.2.2 Review of Customer Requirements ................................................................................................ 12 7.2.3 Customer Communication ............................................................................................................... 12 7.3 Design and Development ........................................................................................................................ 13 7.4 Purchasing .............................................................................................................................................. 13 7.5 Provision of [Production of adhesive tape] .............................................................................................. 13 7.5.1 Control of Provision of [Production of adhesive tape] ..................................................................... 13 7.5.2 Identification and Traceability .......................................................................................................... 14 7.5.3 Property Belonging to Third Parties ................................................................................................ 14 7.5.4 Preservation .................................................................................................................................... 14 7.5.5 Post-Delivery Activities .................................................................................................................... 14 7.5.6 Process Change Control ................................................................................................................. 15 7.5.7 Measurement and Release of [Production of adhesive tape] ......................................................... 15 7.5.8 Control of Nonconforming Outputs .. (15)
质量手册翻译中英文术语表 3.1.1 质量 quality 3.1.2 要求 requirement 3.1.3 等级 grade 3.1.4 顾客满意 customer satisfaction 3.1.5 能力 capability 3.2.1 体系(系统) system 3.2.2 管理体系 management system 3.2.3 质量管理体系 quality management syste m 3.2.4 质量方针 quality policy 3.2.5 质量目标 quality objective 3.2.6 管理 management 3.2.7 最高管理者 top management 3.2.8 质量管理 quality management 3.2.9 质量策划 quality planning 3.2.10 质量控制 quality control 3.2.11 质量保证 quality assurance 3.2.12 质量改进 quality improvement 3.2.13 持续改进 continual improvement 3.2.14 有效性 effectiveness 3.2.15 效率 efficiency 3.3.1 组织 organization 3.3.2 组织结构 organizational structure 3.3.3 基础设施 infrastructure 3.3.4 工作环境 '77ork environment 3.3.5 顾客 customer 3.3.6 供方 supplier 3.3.7 相关方 interested party 3.4.1 过程 process 3.4.2 产品 product 3.4.3 项目 project 3.4.4 设计和开发 design and development 3.4.5 程序 procedure
Table of Contents 1. Purpose & Scope (2) 2. Applicable Standards (2) 3. Business Profile (2) 4. Authority & Responsibility (2) 5. Terms & Definitions (2) 6. Policy & Objectives (3) 7. Application (4) 8. Quality Management System (4) 9. Management Responsibility (6) 10. Resource Management (8) 11. Product Realization (8) 12. Purchasing (11) 13. Production Control / Product Identification & Traceability (12) 14. Control of Inspection, Measuring, and Test Equipment (13) 15. Measurement, Analysis, Improvement (14)
1. Purpose & Scope This manual describes the Quality Management System (QMS) established by and for Dongguan XXX Appliances Limited. The principles and policies on which this manual is based; along with operating procedures, work instruc-tions, and other supporting documents; govern all processes that affect quality throughout the organiza-tion. 2. Applicable Standards 2.1 The QMS is structured and intended to be in compliance with the following standards. ISO 13485:2016 Medical Devices Quality Management Systems Requirements for Regulatory Purposes 21 CFR Part 820 Quality System Regulation (Exclusions and Exceptions noted below.) 2.2 Normative References ISO 9000:2015 Quality Management Systems · Fundamentals and Vocabulary ISO 13485:2016 Medical devices · Quality Management Systems · Guidance on the Application of ISO 13485:2016 3. Business Profile 3.1 Mission Statement To deliver zero defects to our internal and external suppliers and customers. 3.2 XXX, with one facility located at xxx, Dongguan City, Guangdong Province, China. 3.3 The organizational structure is described by Dongguan XXX Appliances Limited Organization Chart. 4. Authority & Responsibility 4.1 This manual is issued under the authority of the President. 4.2 It is the responsibility of the Director of Quality, who is the designated Management Representative, to ensure that the principles of this manual, the Quality Policy, quality objectives, customer requirements, applicable regulatory requirements, and quality management system requirements are promoted, com-municated to and understood by all XXX employees. 5. Terms & Definitions 5.1 Corrective Action A process improvement methodology aimed at identifying and eliminating the causes of known non-conformities to prevent their recurrence. A problem solving process.
ISO 9001:2000 QUALITY MANUAL WOLSTENHOLME INTERNATIONAL LIMITED CONTENTS 1.0 Scope of the Quality Management System at Wolstenholme International Limited 1.1 Statement of Quality Policy 2.0 Quality Management System Documented Procedures 2.1 Document Control 2.2 Control of Quality Records 2.3 Internal Quality Audits 2.4 Control of Nonconforming Product 2.5 Corrective Action 2.6 Preventative Action 3.0 Quality Management System Processes and Interrelations 3.1 Organisation Charts 3.2 Responsibilities 3.3 Interrelation of Processes 3.4 Processes 1.0 Scope of the Quality Management System at Wolstenholme International Limited
Wolstenholme International Limited is a Company with a long-standing successful history of supplying metal powders, pastes, pellets, varnish, offset ink, flexography ink and other related products for use in a large range of applications, on a worldwide basis. The scope of the Quality Management System encompasses all activities on the Darwen Site. The Metallic Powders Operation at Darwen is responsible for the manufacture and supply of metal powders and pigments mainly in aluminium, copper and brass (bronze) based alloys, for use in printing, inks, plastics and other industrial and engineering applications. The Ink Operation at Darwen is responsible for the manufacture of a wide range and type of printing ink, 'one-pack' gold ink, varnish and related printing products. All production processes at Darwen are validated before leaving the site and as such sub-clause 7.5.2 is excluded. The overall operation at Darwen is controlled by the Ink & Print business unit and the Industrial business unit. Technical Service and Research and Development are vital to the future development of the Company, together with understanding and developing new products and applications for our customers. The manufacturing, technical and administration functions of the Company are based in Darwen, Lancashire, England and employ some 210 staff at that location. Through the application of Quality Management Systems and Company wide training programmes, Wolstenholme International Ltd. is committed to ensuring continuous improvements to both its processes and product technology.
Quality Management System Policy Manual ISO 9001:2015 11-8-17 Date Printed: _______________
1. Quality Management System Scope COMPANY NAME establishes this quality policy manual to implement and maintain a quality management system meeting the requirements of ISO 9001:2015, to ensure customer satisfaction in the manufacturing of stamped, formed, machined and fabricated metal parts, weldments, subassemblies and painting of metal parts to customer and COMPANY NAME specifications 1.1. Non-applicable Clauses of ISO 9001: 1.1.1. 8.3 Product Design & Development of Products & Services – COMPANY NAME is a custom manufacturer and designs are provided by our customers. 2. Quality Policy Statement COMPANY NAME is committed to continually improving all products and services to achieve our customer’s expectations. We do this by: 1) Living our values, 2) Providing opportunities for employee involvement, motivation and training, 3) Developing, documenting and following processes. 3. Quality Objectives COMPANY NAME’s quality management system objectives are to enable COMPANY NAME to be our customers’ first choice by: 1) Achieving satisfactory ratings on quality, delivery and other key metrics tracked and reported by our customers through their formal supplier evaluation and performance systems, 2) Achieving a level of 700 ppm as tracked through COMPANY NAME’s RA system for customers without a formal supplier evaluation system for quality, 3) Achieving a 95% on-time delivery to the COMPANY NAME warehouse for customers without a formal supplier evaluation system for delivery. Policy Manual Revisions Log Date 3/22/17 8/7/17 Summary of Revisions Rewrite of Quality Policy Manual to meet ISO 9001:2015. 1. Revised 4.2 to include “employees”, “owners” and “regulators”. Made By JGV JGV 2. Added Figure 2 to support to support the COMPANY NAME QMS Process Flow 11-8-17 Updated to clarify risk, inputs, out puts and KPI’s for Support and Management Process in QMS Process Reference page (page 6) JGV Management Approval Name: Title: Signature: Date: Paul Gintner President
Quality Manual Reference Standard ISO/IEC 17025:2017 Issue Number 01 Issue Date 01–08–2018 Copy Number Total Pages Issued To Address Prepared By Approved By Issued By Name Designation Quality Manager CEO Quality Manager Prepared By Approved By Issued By Page 1 of 59
Prepared By Approved By Issued By Page 3 of 59 Chapter → 1.0 General Information 1.1 Table of contents Chapter No. Subject Amend ment No. Page No. ISO/IEC 17025 Clause Ref. 1 Cover page, table of contents, amendment record sheet and glossary of terms (abbreviation) 00 1 – 6 ========== 2 Authorization statement and laboratory profile and context of organization 00 7 – 9 ========== 3 Control and distribution 00 10 – 11 ========== 4.0 General requirements 4.1 Impartiality 00 12 – 13 4.0 4.2 Confidentiality 00 14 5.0 Structural requirements 00 15 – 20 5.0 6.0 Resource requirements 6.0 6.1 General 00 21 6.2 Personnel 00 21 – 22 6.3 Facilities and environmental conditions 00 23 6.4 Equipment 00 24 – 26 6.5 Metrological traceability 00 27 6.6 Externally provided products and services 00 28 – 29 7.0 Process requirements 7.0 7.1 Review of requests, tenders and contracts 00 30 – 31 7.2 Selection, verification and validation of methods 00 32 – 34 7.3 Sampling 00 35 7.4 Handling of test or calibration items 00 36 7.5 Technical records 00 37 7.6 Evaluation of measurement uncertainty 00 38 7.7 Ensuring the validity of results 00 39 – 40 7.8 Reporting of results 00 41 – 43 7.9 Complaints 00 44 7.10 Nonconforming work 00 45 7.11 Control of data – Information management 00 46