
Spawning time,spawning frequency and fecundity of Japanese chub mackerel,Scomber japonicus in the waters around the Izu Islands,Japan
Tomohide Yamada a ,Ichiro Aoki a,*,Isamu Mitani b
a
Department of Aquatic Bioscience,Graduate school of Agricultural and Life Science,The University of Tokyo,Bunkyo,Tokyo 113,Japan
b
Kanagawa Prefectural Fishery Experimental Station,Youroushi,Jogashima,Misaki,Kanagawa 238-02,Japan
Received 4July 1997;accepted 5April 1998
Abstract
Female Japanese chub mackerel,Scomber japonicus ,were collected in 1993from April to June (36days),in the water around Izu Islands,Japan,which is one of the primary spawning areas.Spawning time,spawning frequency and batch fecundity were determined by histological methods.Temporal frequency of hydrated oocytes and new postovulatory follicles showed that female chub mackerel spawned actively from 22.00to 24.00hours.The average spawning frequency was 17.4%during this sampling period.We estimated that chub mackerel spawned every 5.7days (6.3times)during these 36days.Fifteen females spawned almost every day.Twelve females which had hydrated oocytes in their ovaries were used for estimating batch fecundity.The average batch fecundity was 89200oocytes per female,and the relative batch fecundity was 158eggs per gram female wet weight.The batch fecundity was signi?cantly correlated with condition factor.This shows that batch fecundity was affected by nutritional state of spawning female.#1998Elsevier Science B.V .All rights reserved.
Keywords:Japanese chub mackerel;Batch fecundity;Postovulatory follicle;Spawning frequency;Spawning time;Ovary histology
1.Introduction
Japanese chub mackerel (Scomber japonicus )is one of the most important ?shery stocks in Japan.The size of this stock increased in the 1960s and remained high in the 1970s.The stock,however,decreased continu-ously in the 1980s and is currently at a low level.Spawning dynamics is a fundamental element in assessing and managing ?sh stocks.Maturation and spawning of Japanese chub mackerel have been
reported around the Izu Islands,Japan,which appears to be one of the main spawning grounds (Murayama et al.,1995;Yamada et al.,1996).Daily egg produc-tion methods (DEPM)(Alheit,1993)may be applied to estimate the spawning biomass of chub mackerel populations.Priede and Watson (1993)suggested that DEPM should be preferred for estimation of biomass in Atlantic mackerel (Scomber scombrus ).By this method,the spawning frequency de?ned as the ratio of the number of females and the batch fecundity as the number of eggs released per spawning,are essen-tial parameters.The spawning frequency and batch fecundity of chub mackerel have been reported
only
*Corresponding author.Tel.:+81338122111,ext.5307;fax:+81338120529;e-mail:aoki@hongo.ecc.u-tokyo.ac.jp 0165-7836/98/$±see front matter #1998Elsevier Science B.V .All rights reserved.P I I :S 0165-7836(98)00113-1
by Dickerson et al.(1992)for the population off the west coast of North America.Similar data do not exist for the Japanese population located in the north-western Paci?c Ocean.
The annual egg production method has been applied to the Japanese chub mackerel on the basis of the number of oocytes greater than 0.5mm diameter in the ovary (Watanabe,1983).However,serial spawners such as chub mackerel do not have annual egg pro-duction determined prior to the spawning season (Hunter et al.,1985).The objectives of the present study are to apply the DEPM to the Japanese chub mackerel stock,and in addition to investigate spawn-ing time,spawning frequency and batch fecundity of this species around the Izu Islands.2.Materials and methods 2.1.Sampling
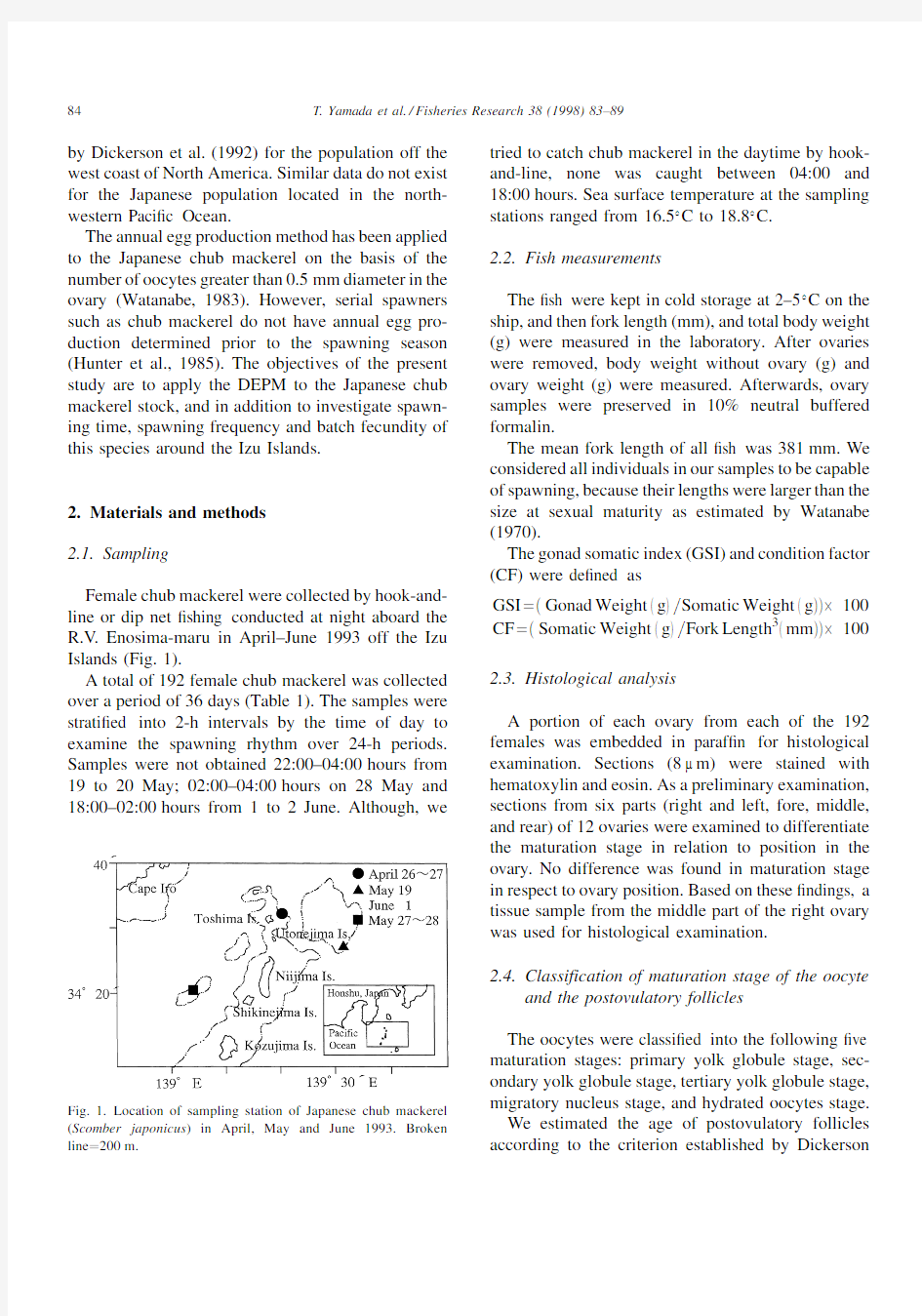
Female chub mackerel were collected by hook-and-line or dip net ?shing conducted at night aboard the R.V .Enosima-maru in April±June 1993off the Izu Islands (Fig.1).
A total of 192female chub mackerel was collected over a period of 36days (Table 1).The samples were strati?ed into 2-h intervals by the time of day to examine the spawning rhythm over 24-h periods.Samples were not obtained 22:00±04:00hours from 19to 20May;02:00±04:00hours on 28May and 18:00±02:00hours from 1to 2June.Although,we
tried to catch chub mackerel in the daytime by hook-and-line,none was caught between 04:00and 18:00hours.Sea surface temperature at the sampling stations ranged from 16.58C to 18.88C.2.2.Fish measurements
The ?sh were kept in cold storage at 2±58C on the ship,and then fork length (mm),and total body weight (g)were measured in the laboratory.After ovaries were removed,body weight without ovary (g)and ovary weight (g)were measured.Afterwards,ovary samples were preserved in 10%neutral buffered formalin.
The mean fork length of all ?sh was 381mm.We considered all individuals in our samples to be capable of spawning,because their lengths were larger than the size at sexual maturity as estimated by Watanabe (1970).
The gonad somatic index (GSI)and condition factor (CF)were de?ned as
GSI Gonad Weight g a Somatic Weight g ?100CF Somatic Weight g a Fork Length 3 mm ?1002.3.Histological analysis
A portion of each ovary from each of the 192females was embedded in paraf?n for histological examination.Sections (8m m)were stained with hematoxylin and eosin.As a preliminary examination,sections from six parts (right and left,fore,middle,and rear)of 12ovaries were examined to differentiate the maturation stage in relation to position in the ovary.No difference was found in maturation stage in respect to ovary position.Based on these ?ndings,a tissue sample from the middle part of the right ovary was used for histological examination.
2.4.Classification of maturation stage of the oocyte
and the postovulatory follicles The oocytes were classi?ed into the following ?ve maturation stages:primary yolk globule stage,sec-ondary yolk globule stage,tertiary yolk globule stage,migratory nucleus stage,and hydrated oocytes stage.We estimated the age of postovulatory follicles according to the criterion established by
Dickerson
Fig.1.Location of sampling station of Japanese chub mackerel (Scomber japonicus )in April,May and June 1993.Broken line 200m.
84T.Yamada et al./Fisheries Research 38(1998)83±89
et al.(1992):0day(0±24h after spawning),1day (24±48h after spawning),2days(over48h after spawning).
We estimated the spawning time and spawning frequency by classifying reproductive states of females from the maturation stage of oocyte and age of the postovulatory follicle in ovaries,based on female samples of2-h segments.
The reproduction states of females were classi?ed into the followings.
1.Migratory nucleus oocytes:<24h prior to spawn-ing.
2.Hydrated oocytes:spawning imminent.
3.Hydrated oocytes and new postovulatory follicles: in the act of spawning.
4.0day postovulatory:<24h after spawning.
5.1day postovulatory follicles:24±48h after spawn-ing
6.2days postovulatory follicles:>48h after spawn-ing.
2.5.Estimation of batch fecundity
Batch fecundity was estimated according to the method of Hunter et al.(1985),which was based on the number of hydrated oocytes in the ovary.The number of hydrated oocytes was counted using a microscope.Females that had both new postovulatory follicles and hydrated oocytes at the same time were not appropriate for batch fecundity estimation, because such females were considered to have begun to spawn the time of sampling.These specimens were excluded from the batch fecundity estimation.Thus, only12females were available for the estimation of the batch fecundity.
3.Results
3.1.Spawning time
Temporal patterns of reproductive states of females are illustrated in Fig.2.The frequencies of occurrence of the hydrated occytes were12%and15%for18:00±20:00hours and20:00±22:00hours,respectively,but they decreased to3%for22:00±24:00hours and to zero after24:00hour.In contrast,while the0day postovulatory follicles occurred about6%between 20:00and22:00hours,they appeared in30%of females after22:00hour.Females having both hydrated oocytes and new postovulatory follicles were found for20:00±02:00hours,though the percentage was small.The frequency of the individuals that had1
Table1
Number of samples,sea surface temperature(SST)of sampling stations,and mean fork length(FL)?standard deviation(SD)
Date Sampling time(h)
18±2020±2222±240±22±4
SST(8C)16.516.717.317.217.1
Mean FL?SD(mm)345?29381?16382?21381?18389?24 19May Number9*13*
SST(8C)17.918.3
Mean FL?SD(mm)365?28378?15
27±28May Number1615*1514*
SST(8C)17.217.617.117.2
Mean FL?SD(mm)390?22383?17378?27383?13
1June Number18* SST(8C)18.8
Mean FL?SD(mm)384?15 Total Number3347403438 FL?SD(mm)374?30381?16381?23382?16386?20
GSI?SD8.14?3.018.89?3.628.49?3.009.07?2.558.35?2.23
CF?SD11.17?0.7411.13?0.8011.21?0.8211.33?0.6410.81?0.75 Fish were caught by hook-and-line and dipnet,but in several cases(*)only by hook-and-line.Blank columns no samples.Means of fork length,gonadosomatic index(GSI)and condition factor(CF)are also shown for2h sampling segments
T.Yamada et al./Fisheries Research38(1998)83±8985
day or 2day postovulatory follicles was independent of the time of day,about 10%.There were no sig-ni?cant differences in fork length,GSI and CF among female samples from different times of day (ANOV A,P <0.05).Spawning of the female started at around 20:00hour,with spawning activity peaking between 22:00and 24:00hours,and completed by 02:00hour.3.2.Spawning frequency
The spawning frequency of a serial spawner was de?ned as the ratio of spawning females to all females (Hunter and Macewicz,1980).The average spawning frequency of the chub mackerel was estimated from the three independent ratios of spawning females:(1)the ratio of the females with the migratory oocytes,(2)the ratio of females with the hydrated oocytes and the new postovulatory follicles,(3)the ratio of the female with the 1day postovulatory follicles.
While the average spawning frequencies on 26±27April and 19May were 8.0%and 7.6%,those on 27±28May and 1June were about 30%(Table 2).This shows seasonal variability of the spawning frequency.The average spawning frequency of all females during the entire sampling period (36days)was estimated to be 17.4%.Hence,female chub mackerel spawned on the average every 5.7days and 6.3times during those 36days.
It was noted that 15females had migratory nucleus oocytes,hydrated oocytes,and postovulatory
follicles
Fig.2.Temporal changes in frequencies of the females with migratory nucleus oocytes (MN),hydrated oocytes (HO),and postovulatory follicles within 0±24h after spawning (PO a ),24±48h after spawning (PO b ),and 48h after spawning (PO c ).HO PO a indicates females with both hydrated oocytes and postovulatory follicles.
Table 2
Spawning frequencies of chub mackerel females,which were estimated from percentages of females in the three spawning states Date Number of mature females Spawning per day (%)MN HO,HO PO a ,PO a PO b Mean 19May 22 4.69.19.17.627±28May 6021.751.718.330.61June 1827.844.427.833.3All
192
15.1
26.6
10.4
17.4
MN:Migratory nucleus oocyte.HO:Hydrated oocyte.
PO a :Postovulatory follicles within 24h after spawning.PO b :Postovulatory follicles 24±48h after spawning.HO PO a :In the act of spawning.
86T.Yamada et al./Fisheries Research 38(1998)83±89
at the same time (Table 3).Twelve of these 15females had spawned on 2or 3successive days.The mean spawning interval was 1.20days during these 36days (Table 3).
3.3.Batch fecundity
Most of the samples used for estimating the batch fecundity were collected between 18:00and 22:00hours.Batch fecundity showed a wide variation among females.The maximum and minimum was 157600and 18900,respectively,while the average was 89200(Table 4).Relative batch fecundity was de?ned as,batch fecundity per gram female body
weight without ovary.The average relative batch fecundity was calculated to be 158.The average fecundity per female during the 36day sampling period was estimated at 562000(spawning times (6.3)?average batch fecundity (89200)).Although there was no signi?cant correlation between batch fecundity and fork length (r 0.219,P >0.05)and body weight (r 0.479,P >0.05),batch fecundity was clearly correlated with condition factor (r 0.721,P <0.05)(Fig.3).4.Discussion
4.1.Spawning in relation to time of day
In our study,the state of oocytes changed and the frequency of females with new postovulatory follicles
Table 3
Numbers of chub mackerel females with migratory nucleus oocytes,hydrated oocytes,and postovulatory follicles simulta-neously,and mean spawning intervals of these females Spawning state Day interval between spawning (i )Number of
females (N )PO a PO b 14MN PO a
14MN PO a PO b 13HO PO b 11MN PO b 23
Average
iN /
N 18/15 1.2
MN:Migratory nucleus oocyte.HO:Hydrated oocyte.
PO a :Postovulatory follicles within 24h after spawning.PO b :Postovulatory follicles 24±48h after spawning.
Table 4
Batch fecundity of chub mackerel females (n 12)with hydrated oocytes Date Fork length (mm)Body weight without ovary (g)Ovary weight (g)Batch fecundity Relative batch fecundity 26April 368556.371.08150014626April 371532.266.0733*******May 329366.535.84650012719May 393607.371.87430012227May 369549.794.410220018627May 380636.582.58990014127May 353482.272.182********May 385702.694.915760022427May 388587.748.7189003227May 362514.580.811390022127May 381605.5107.515120025027May 380603.883.079000131Mean
372
562.1
75.7
89200
158
Relative batch fecundity batch fecundity/body weight without
ovary.
Fig.3.Relation between batch fecundity and condition factor (somatic weight (g)/fork length (mm)3)?102.
T.Yamada et al./Fisheries Research 38(1998)83±89
87
increased after22:00hour,while females with hydrated oocytes were not present after24:00hour. Watanabe(1970)collected eggs with a plankton net in time series in the spawning grounds,and estimated that spawning of chub mackerel gradually started after sunset with peak spawning activity between22:00and 24:00hours,so our results are consistent with his. The Japanese sardine,Sardinops melanostictus, spawns primarily between20:00and23:00hours (Matsuura,1992;Morimoto,1993;Murayama et al., 1994).The Japanese anchovy,Engraulis japonicus, discharges eggs actively between22:00and 24:00hours(Takano et al.,1983;Turuta,1992).Thus, the spawning times of such small pelagic?shes are similar.This may be a mechanism to reduce predation on newly spawned eggs by zooplanktivores.More-over,as adults are off their guard when spawning, night spawning could also insure the safety of small ?shes.
4.2.Spawning frequency
The histological results revealed seasonal changes in spawning activity.The average spawning frequency indicated that females spawned more actively in late May(30.6%)through early June(33.3%),than from late April(8.0%)to mid May(7.6%).
In a study of chub mackerel from the west coast of North America,the average spawning frequency was 8.7%during sampling periods(101days)(Dickerson et al.,1992).Our estimate of the average spawning frequency of17.4%was higher than that of the North America mackerel.Our sample did not cover the entire spawning season and the high frequency was probably caused by our sampling period being concentrated at the peak of spawning.The spawning frequency may be low at the beginning and end of spawning season. Dickerson et al.(1992)reported that32of271 females spawned every1.3days.Similarly,15females of192chub mackerel females in our study spawned every1.2days.Daily spawning appears to occur at the peak of the spawning season.
The spawning frequency estimation of Hunter and Goldberg(1980)is based on the assumption that a spawning population does not move in and out of the sampling area and during the spawning season. Although,we did not investigate the movement of the spawning?sh,in some tagging experiments,chub mackerel rarely moved within the spawning area in the peak of spawning season(I.Mitani,unpublished data). It has been reported that the disappearance time of the postovulatory follicles may be inˉuenced by ambient water temperature(Goldberg et al.,1984; Hunter and Macewicz,1985;Turuta,1992).The dragonet,Callionymus enneactiis,spawns every day at28±308C and postovulatory follicles of this species are not seen in ovaries15h after spawning(Takita et al.,1983).In the Japanese sardine,Sardinops malanostictus,postovulatory follicles are not seen 38h after ovulation at208C and48h after spawning at178C(M.Shiraishi,unpublished data).Descriptions by Dickerson et al.(1992)of the change in postovu-latory follicles of chub mackerel were based on rear-ing experiments at208C.In our study,the sea surface temperature in the sampling area was16.5±18.88C. The estimated age of postovulatory follicles may have been a little longer.
4.3.Fecundity
Our estimate of average batch fecundity(89200 oocytes)was greater than that(68400oocytes)of Dickerson et al.(1992).This is due to the difference in body size.The average batch fecundity per gram body weight without ovary in our study(158oocytes/g)was close to(168oocytes/g)in Dickerson et al.(1992). Dickerson et al.(1992)estimated batch fecundity by calculating the migratory nucleus oocytes.The oocytes frequency method usually gives results similar to those based on counts of hydrated oocytes,if females with highly advanced oocytes are used (Hunter and Goldberg,1980;Laroche and Richardson, 1980).
There was a signi?cant correlation between condi-tion factor and batch fecundity.This suggests that nutritional condition affects batch fecundity.One female in our samples had an extremely low fecundity; 18900(relative batch fecundity 32).There were many beta atresia oocytes and no yolked oocytes in this female,whereas,the other females did not have beta atresia oocytes in their ovaries.The presence of beta atresia oocytes and the absence of yolked oocytes indicate the end of spawning(Dickerson et al.,1992). Therefore,this female had most likely completed spawning for the season.The CF value of this female was the lowest of all the females.The batch fecundity
88T.Yamada et al./Fisheries Research38(1998)83±89
of the female was low possibly due to the consumption of nutrition by repeated times of spawning.For Atlan-tic mackerel,as spawning?sh migrate northwards the batch size decreases with progress of the spawning season(Watson et al.,1992).Thus,there may be a difference in batch fecundity between the start and end of the spawning season.
In this study,only12females were sampled.More samples are needed to estimate more reliably batch fecundity.As batch fecundity in chub mackerel is likely to vary annually,it should be estimated annually in relation to appropriate environmental factors.
Acknowledgements
We would like to thank Dr.Manabu Shiraishi of the National Research Institute of Aquaculture,Dr. Nobuyuki Azuma of Hirosaki University,the captain and crew of the Enoshimamaru,and the staffs of Kanagawa Prefectural Fisheries Experiment Station.
References
Alheit,J.,https://www.doczj.com/doc/2d6975279.html,e of the daily egg production method for estimating biomass of clupeoid fishes:a review and evaluation.
Bull.Mar.Sci.53,750±767.
Dickerson,T.,Macewicz,B.J.,Hunter,J.R.,1992.Spawning frequency and batch fecundity of chub mackerel Scomber japonicus1985.CalCOFI Rep.33,130±140. Goldberg,S.R.,Alarcon,V.H.,Alheit,J.,1984.Postovulatory follicle histology of the Pacific sardine Sardinops sagax,from Peru.Fish.Bull.U.S.82,443±445.
Hunter,J.R.,Goldberg,S.R.,1980.Spawning incidence and batch fecundity in northern anchovy Engraulis mordax.Fish.Bull.
U.S.77,641±652.
Hunter,J.R.,Macewicz, B.J.,1980.Sexual maturity,batch fecundity,spawning frequency and temporal pattern in the northern anchovy,Engraulis mordax,during1979spawning season.CalCOFI Rep.21,139±149.
Hunter,J.R.,Lo,N.C.H.,Leong,R.J.H.,1985.Batch fecundity in multiple spawning fishes.NOAA Tech.Rep.NMFS.36,67±77. Hunter,J.R.,Macewicz,B.J.,1985.Measurement of spawning frequency in multiple spawning fishes.NOAA Tech.Rep.
NMFS.36,79±https://www.doczj.com/doc/2d6975279.html,roche,J.L.,Richardson,S.L.,1980.Reproduction of northern anchovy,Engraulis mordax,off Oregon and Washington.Fish.
Bull.U.S.78,603±618.
Matsuura,S.,1992.Reproductive cycle of sardine.Kaiyo(Marine Science)24,289±294(in Japanese).
Morimoto,H.,1993.Time of maximal oocyte hydration and spawning in the Japanese sardine in Tosa Bay,southwestern Japan.Nippon Suisan Gakkaishi59,7±14(in Japanese,with English abstract).
Murayama,T.,Shiraishi,M.,Aoki,I.,1994.Change in ovarian development and plasma levels of sex steroid harmones in the wild females Japanese sardine(Sardinops melanostictus) during the spawning period.J.Fish Biol.45,235±245. Murayama,T.,Mitani,I.,Aoki,I.,1995.Estimation of the spawning period of the Pacific mackerel Scomber japonicus based on the changes in gonad index and the ovarian histology.
Bull.Jpn.Soc.Fish.Oceanogr.59,11±17(in Japanese,with English abstract).
Priede,I.G.,Watson,J.J.,1993.An evaluation of the daily egg production method for estimating biomass of Atlantic mackerel (Scomber scombus).Bull.Mar.Sci.53,891±911. Takano,K.,Kishida,T.,Ueda,K.,1983.Number of eggs of Japanese anchovy produced per female per year estimated by a rearing experiment.Bull.Nansei https://www.doczj.com/doc/2d6975279.html,b.15,1±11 (in Japanese,with English abstract).
Takita,T.,Iwamoto,T.,Kai,S.,Sogabe,I.,1983.Maturation and spawning of the Dragonet,Callionymus enneactis,in an aquarium.Jpn.J.Ichthyol.30,221±226.
Turuta,Y.,1992.Reproduction in the Japanese anchovy(Engarulis japonica)as related to population fluctuation(in Japanese,with English abstract).Bull.Nat.Res.Inst.Fish.Eng.13,129±168.
Watanabe,T.,1970.Morphology and ecology of early stages of life in Japanese common mackerel,Scomber japonicus Houttuyn, with special reference to fluctuation of population.Bull.Tokai https://www.doczj.com/doc/2d6975279.html,b.62,1±283(in Japanese,with English abstract).
Watanabe,T.,1983.Spawning survey method.In:Ishii,T.(Ed.), Population Dynamics of Fishery Resources,Koseisha-koseikaku, Tokyo,pp.9±29(in Japanese).
Watson,J.J.,Priede,I.G.,Witthames,P.R.,Owari-Wadunde,A., 1992.Batch fecundity of Atlantic mackerel Scomber scombrus L.J.Fish Biol.40,591±598.
Yamada,T.,Aoki,I.,Shiraishi,M.,Mitani,I.,1996.Maturation and spawning of the Japanese chub mackerel,Scomber japonicus,in the sea area of Izu Islands.Bull.Jpn.Soc.
Fish.Oceanogr.60,331±338(in Japanese,with English abstract).
T.Yamada et al./Fisheries Research38(1998)83±8989
第三章鱼类人工繁殖的生物基础 第一节鱼类的性腺发育规律 一、生殖细胞的发育和成熟 二、卵巢、精巢的形态结构和分期 三、鱼类的性成熟的年龄和性周期 第二节中枢神经与内分泌系统在鱼类繁殖中的作用 一、中枢神经系统在鱼类繁殖中的作用 二、内分泌系统在鱼类繁殖中的作用 第三节环境因素对鱼类性腺发育的影响 一、营养 二、温度 三、光照 四、水流 五、盐度 第一节鱼类的性腺发育规律 鱼类人工繁殖的成效主要取决于性腺发育状况。性腺发育的全过程直接和间接地受内分泌腺及神经系统的控制。鱼类的性腺由体腔背部2个隆起嵴(生殖褶)发育而成。生殖褶由上皮细胞转化为原始性细胞时,分不出雌雄;进一步分化成卵原细胞和精原细胞后,以不同的方式发育成卵子或精子。鱼类性腺的发育进程主要由卵子和精子的发生过程决定。 一、生殖细胞的发育和成熟 (一)鱼类卵细胞的发育与成熟 1、卵原细胞分裂期卵原细胞反复进行有丝分裂,细胞数目不断增加,经过若干次分裂后,卵原细胞停止分裂,开始生长,向初级卵母细胞过渡。此阶段的卵细胞为第Ⅰ时相卵原细胞,以第Ⅰ时相卵原细胞为主的卵巢称第Ⅰ期卵巢。 2、卵母细胞生长期此期分为小生长期和大生长期。 小生长期是卵母细胞的生长期,开始时,细胞质呈微粒状,细胞核卵形,占卵母细胞的大部分,其内壁四周排列着许多小核(或称核仁),中央为粒状的染色质,有时细胞质中可见卵黄核。卵母细胞进一步发育,卵膜外出现了一层滤泡膜,由单层上皮细胞组成,内有长形的核。小生长期发育到单层滤泡为止,这时的卵母细胞,称为卵母细胞成熟的第Ⅱ时相,以第Ⅱ时相卵母细胞为主的卵巢称为Ⅱ期卵巢。性未成熟的鱼,常有相当长的时期停留在Ⅱ期。 大生长期是营养物质生长的阶段。卵母细胞由于卵黄及脂肪的积贮而体积大大增加。卵黄沉积可分2个阶段:①卵黄开始沉积阶段。卵膜变厚,出现放射状纹。滤泡膜的上皮
鱼类的生殖 l.鱼类性成熟的年龄 鱼类性成熟指鱼类从孵化后经生长发育,性腺达到成熟能排精产卵。不同鱼类性成熟开始年龄不相同。即使同一种鱼,也因外界环境条件的差异,性成熟的年龄也有变化。此外,同一种鱼的不同种群或雌雄两性到达性成熟的年龄也有差异。 一般说来,分散生活的种类,性成熟的年龄较迟。如国家珍贵保护动物中华鲟,开始性成熟的年龄是4龄。欧洲鳇鱼性成熟时间为16龄。集群生活的鱼类,性成熟时间稍早,如中上层生活的青鳞小沙丁鱼和金色小沙丁鱼,1龄即达性成熟,而中下层的大黄鱼性成熟时间大多在3~4龄。也有一些更早熟的如食蚊鱼,出生后1个月即能产卵。同一种鱼,在低纬度的南方比在高纬度的北方性成熟早。如鲤鱼,在南方2龄性成熟,而在北方则要5龄才能性成熟。鲻鱼、梭鱼的雌鱼在4龄开始性成熟,而雄鱼则在2~3龄性成熟。有些鱼类的性成熟是同步的,如大银鱼,1龄全部性成熟,且雌雄同步。而有些鱼类,如黄海的鲷鱼,1龄时只有4%雌鱼性成熟,雄鱼45%性成熟,2龄鱼全部性成熟。近年来的研究表明,鱼类资源遭受过度捕捞时,鱼类趋向小型化,性成熟年龄也提早。 2.鱼类性成熟的第二性征 有些鱼类可以从外部形态特征区别雌雄,如身体大小、体色、泄殖孔向外开口等。又如板鳃鱼类的鳍脚,鳉鱼雄鱼的交接器,鳑鱼的产卵管等。它们的两性异形是稳定的。大多数鱼类只能从其内部的精巢和卵巢区别其雌雄,而不易从外形上区别。不过有些鱼类在性成熟的繁殖季节出现第二性征。如鲤科鱼类中,有些种类雄鱼身体各部多具鲜明色彩,而雌鱼多呈暗淡的灰黄色。粘皮虎鱼的雄鱼深灰色,两背鳍皆较大,第一背鳍上有黄色和黑色斑块各一个。腹鳍吸盘宽圆,棕黑色。雌鱼灰黄色,第一背鳍上有黑色条纹,第二背鳍上也有黑色条纹。腹鳍吸盘较窄,乳白色。海洋中的隆头鱼,雄鱼橙黄色,自眼部向后有5~6条蓝色条纹,而雌鱼为红色,没有条纹。 许多鱼类在繁殖季节身体出现鲜红的色彩,或原来的色彩变得更加鲜艳。一般在雄鱼中表现特别突出。生殖季节过后,色彩即行消失,这种色彩称婚姻色。
海水鱼类人工配合饲料的营养与特性 1.钙一般在淡水鱼的预混料中钙的含量较多,因为淡水中溶解的钙少,而海水中溶解的钙则趋于饱和,所以海水鱼中钙的补充就少。事实上,如果过多的钙进人鱼体,超过肾功能的负荷,就会抑制生长,所以在借用淡水鱼预混料配方时,要根据养殖海水盐度的高低作适当的调整,高盐度应少添加,低盐度应多添加,但不管水质如何变化,钙对鱼类生长发育是必不可少的。 2.磷磷在水中基本上是一种限制因子。水环境中能提供的量有限,所以大部分需要从饵料中获得。分析结果证明,海水鱼组织中的磷含量比一般陆生动物和淡水鱼类要高得多,这种生理生化上的差异,一定要引起我们的注意。显然在海水色的预混料中应多添加磷,但是过多的磷又会影响钙的吸收,所以对某一个养殖品种的钙磷需求和钙磷比一直是引起关注的。另外,磷在不同品种的海水鱼中代谢吸收也不同,如大洋性鱼类狮鱼、鲸鱼等,代谢的速度比一般底栖鱼类蝶、纣、石斑鱼要快得多,因此在配制牙解和石斑鱼的预混料时,与大洋性鱼类相比,磷含量就要减少,否则就会影响鱼的生长。 3.铁 从海水鱼生态环境来看,海水中铁的含量不多,因为偏碱性。至于淡水中铁的含量有多有少,则需看具体水质。因此,一般淡水鱼的铁元素补充多少,影响不大,但是海水鱼的需要量应该满足,当然不同种的鱼需求量也不同,过量添加除成本提高外,也会造成代谢及消化吸收上的障碍,从生理上看,大洋性海水鱼的肌红素含量需求较大,比底栖性鱼类要高得多,每一个肌红素中都含有一个铁原子,因此在矿物质中铁的添加量要高,如果使用这样的配方来饲养沿岸性鱼类就应降低铁的添加量。 其它许多种元素,如铜、锌、铝、镁、钾、钠、碘、硒等,都各有不同的生理作用,添加量适宜就会促进生长,反之就会抑制生长,这些还需要进行大量的研究工作。 五、从生理生态及加工过程中对维生素的需求维生素是有机化合物,不同于氨基酸、糖类和脂肪,维生素需要量甚微。动物从外界(经常是饵料)摄人维生素以维持正常生长、繁殖和健康。维生素分为水溶性维生素和脂溶性维生素两大类。八种水溶性维生素需求量相对较少,其主要作为辅酶,被叫做 B 族维生素。另外三种水溶性维生素即胆碱、肌醇和维生素C,其需要量较大,虽不作辅助酶,但具其它功能。维生素儿
《鱼类组织胚胎学》试卷(A)参考答案班级姓名序号分数 一、名词解释(每题 4 分,共 20 分): 1、尼氏体— 2、肌纤维— 3、肠绒毛— 4、肾小球— 5、合子— 二、填空题(每空0、5分,共 15 分): 1、衬贴在心血管及淋巴管内表面的单层扁平上皮称,分布在浆膜表面的单层扁平上皮称为; 2、结缔组织有、、等三种纤维成分;
3、肌组织可分为、和三种类型; 4、鱼的辅助呼吸主要有_ _ _,_ _,_ _, ; 5、消化管壁分四层,即 _ , _,_ ,_ ; 6、胃腺中分泌胃蛋白酶原的细胞是,分泌盐酸的细胞是; 7、胰腺是既有外分泌腺,又有内分泌腺的一种混合腺体,其中主要部分呈管泡状结构,称胰腺腺泡,为,另有一些呈岛屿分布,称胰岛,为; 8、肾单位由_ _、__ _组成,肾小体有两个极,即 _ _、 _ ,前者是 _出入处, 后者与 _ 相连; 9、内分泌腺没有__ _,其分泌物(激素)由腺上皮分泌后直接进入_ _; 10、生殖细胞在成熟期内要经过两次成熟分裂,其中第一次成熟分裂为,第二次成熟分裂。 三、判断题(正确的划“√”,错误的划“×”,每题2分,共20分):
1、肠粘膜柱状上皮间有一种杯状细胞,又称粘液细胞,属于一种单细胞腺体;() 2、由内外分泌腺所分泌的分泌物称为激素;() 3、哺乳动物成熟红细胞无细胞核,鱼类红细胞也无细胞核;() 4、骨骼肌与心肌都有横纹,都属于随意肌;() 5、血管的管壁都是由内膜、中膜和外膜三层结构组成;() 6、微绒毛是指肠粘膜上皮和粘膜固有层向肠腔内折起形成的指状突起;() 7、腮小片是腮丝表面形成的微细的片状突起,为鱼类气体交换的基本单位;() 8、肾小体是由肾小球和肾小管组成;() 9、精子的两次成熟分裂都是在精巢的精小囊内进行;() 10、血浆是由血清和纤维蛋白原组成;() 四、选择题(每题1分,共10分): 1.假复层柱状纤毛上皮分布于:() A.气管;B.食管;C.膀胱; D.肠管。 2.血液中数量最多的白细胞是:()
Spawning time,spawning frequency and fecundity of Japanese chub mackerel,Scomber japonicus in the waters around the Izu Islands,Japan Tomohide Yamada a ,Ichiro Aoki a,*,Isamu Mitani b a Department of Aquatic Bioscience,Graduate school of Agricultural and Life Science,The University of Tokyo,Bunkyo,Tokyo 113,Japan b Kanagawa Prefectural Fishery Experimental Station,Youroushi,Jogashima,Misaki,Kanagawa 238-02,Japan Received 4July 1997;accepted 5April 1998 Abstract Female Japanese chub mackerel,Scomber japonicus ,were collected in 1993from April to June (36days),in the water around Izu Islands,Japan,which is one of the primary spawning areas.Spawning time,spawning frequency and batch fecundity were determined by histological methods.Temporal frequency of hydrated oocytes and new postovulatory follicles showed that female chub mackerel spawned actively from 22.00to 24.00hours.The average spawning frequency was 17.4%during this sampling period.We estimated that chub mackerel spawned every 5.7days (6.3times)during these 36days.Fifteen females spawned almost every day.Twelve females which had hydrated oocytes in their ovaries were used for estimating batch fecundity.The average batch fecundity was 89200oocytes per female,and the relative batch fecundity was 158eggs per gram female wet weight.The batch fecundity was signi?cantly correlated with condition factor.This shows that batch fecundity was affected by nutritional state of spawning female.#1998Elsevier Science B.V .All rights reserved. Keywords:Japanese chub mackerel;Batch fecundity;Postovulatory follicle;Spawning frequency;Spawning time;Ovary histology 1.Introduction Japanese chub mackerel (Scomber japonicus )is one of the most important ?shery stocks in Japan.The size of this stock increased in the 1960s and remained high in the 1970s.The stock,however,decreased continu-ously in the 1980s and is currently at a low level.Spawning dynamics is a fundamental element in assessing and managing ?sh stocks.Maturation and spawning of Japanese chub mackerel have been reported around the Izu Islands,Japan,which appears to be one of the main spawning grounds (Murayama et al.,1995;Yamada et al.,1996).Daily egg produc-tion methods (DEPM)(Alheit,1993)may be applied to estimate the spawning biomass of chub mackerel populations.Priede and Watson (1993)suggested that DEPM should be preferred for estimation of biomass in Atlantic mackerel (Scomber scombrus ).By this method,the spawning frequency de?ned as the ratio of the number of females and the batch fecundity as the number of eggs released per spawning,are essen-tial parameters.The spawning frequency and batch fecundity of chub mackerel have been reported only *Corresponding author.Tel.:+81338122111,ext.5307;fax:+81338120529;e-mail:aoki@hongo.ecc.u-tokyo.ac.jp 0165-7836/98/$±see front matter #1998Elsevier Science B.V .All rights reserved.P I I :S 0165-7836(98)00113-1
鱼类卵细胞的发育与成熟 1.卵原细胞反复进行有丝分裂,细胞数目不断增加,经过若干次分裂后,卵原细胞停止分裂,开始生长,向初级卵母细胞过渡。此阶段的卵细胞为第I时相卵原细胞,以第I时相卵原细 胞为主的卵巢称第I期卵巢。 2. 小生长期是卵母细胞的生长期,开始时,细胞质呈微粒状,细胞核卵形,占卵母细胞的大部分,其内壁四周排列着许多小核(或称核仁),中央为粒状的染色质,有时细胞质中可见卵黄核。卵母细胞进一步发育,卵膜外出现了一层滤泡膜,由单层上皮细胞组成,内有长形的核。小生长期发育到单层滤泡为止,这时的卵母细胞,称为卵母细胞成熟的第n时相,以第n时相卵母细胞为主的卵巢称为n期卵巢。性未成熟的鱼,常有相当长的时期停留在n 期。 第一时相图(1-3):卵母细胞一般出现在体长为 6.0 cm 以 下的当年生幼鱼卵巢中。生殖上皮具有2—3 层增殖分生而成的密集的卵原细胞, 其大小不 一。胞径3.6—16.0卩m胞核2.5 —10.0卩m,浅紫色,可见交织状粗丝形染色质(图1),附于生 殖上皮的第 1 时相卵母细胞形态不规则, 浅紫色, 具 1 个巨大的深紫色核仁(图2)。基质中第 1 时相的卵母细胞长椭圆形, 质膜外出现一层滤泡膜, 具2— 4 个深紫色滤泡细胞(图3)。 第二时相(4-7) (1)早期图: 卵母细胞排列紧密, 形态不一;多数呈多角形、少数为卵圆形。细胞核透亮, 核中 染色体逐渐解散, 但仍可看到呈细丝状残迹。胞质显嗜碱性, 成细颗粒状分布。核椭圆, 染色浅。核仁大小不一, 数量增多, 一般位于核膜内缘。单层滤泡膜, 5—7 个扁平滤泡细胞围 绕着卵母细胞(图4)。 ⑵中期:卵母细胞一般呈椭圆形,胞质深紫色。核与胞质间形成一条宽为0.2 —0.5卩m勺透明层(图5), 透明层内物质密度很稀, 显得透亮。 (3)晚期:卵母细胞排列松散。一般呈圆球形, 胞质呈弱嗜碱性, 油球数量明显增多, 分布均匀。胞质中出现许多呈网状分布的纤维结构, 在网眼中仍有许多被苏木精染成深紫色的微细颗粒。这时细胞核迅速膨大, 核周透明层变薄渐消失, 核仁明显缩小, 绕核膜边缘分布(图6)。单层滤泡膜中出现微血管(图7)。 第 3 时相(8-13) (1)早期图:卵母细胞梨形,胞质浅紫色,油球均匀分布。核位中央,核仁圆形(图版1-8)。在胞质的皮质部分出现一层松散排列、大小不一的液泡(图版1-9),液泡直径2.0—3.0卩m均匀 分 布着浅紫色内容物,一般被认为是中性粘多糖物质。(2)中期:卵母细胞液泡2-3层,液泡间出现紫红色的微小卵黄粒。随着卵母细胞继续发育,卵黄数量增多,并向核方向逐渐扩散,细胞质向核外周堆集,呈紫色粗粒状。核圆形,核仁大小不均,分布在核膜周边(图版I-10)。滤泡膜分化为两层细胞:内层棒形,长4.0—10.0卩m排列紧密;夕卜层细胞扁平、细长,排列疏松(图版1-11)。⑶晚期:卵母细胞(图版I-12)饱满,细胞核近圆球形,位于卵母细胞中央。核膜略呈波纹状,在核膜内缘存在着十几个到数十个不等的细小核仁,一般未见粗大核仁存在。卵黄颗粒直径0.5—1.0 (im油球数量增多,分布不均匀。胞质的皮质部分首先出现一些染成紫红色的卵黄颗粒,以后卵黄颗粒渐增多,广泛分布于液泡之间的细胞质中。细胞膜中未见放射纹。滤泡膜内层细胞基本呈立方形,卜层细胞仍为扁平状(图版I-13)。 第 4 时相(14-4)
祥解燕鱼的繁殖方法有很多鱼友在网上讨论燕鱼的繁殖,断断续续很不全面,也有一些介绍燕鱼繁殖的文章,也都不太详细,还需要鱼友自己去摸索;也许这样会更有乐趣,但也会带来很多失败的苦恼和遗憾。在这里,我把自己繁殖燕鱼的一些做法和经验写出来,与大家一起讨论,共同提高。并希望能给那些想繁殖燕鱼或屡遭失败的朋友一些帮助,少走一些弯路。一、种鱼首先,我们来谈谈种鱼。选种鱼时,要选健康的、色彩对比强烈艳丽、体形好的小鱼,从小养大。关于“残疾”问题,要一分为二去看,有些残疾是要在选鱼时就坚决避免的,如:身体歪斜,头、咀变形等;但一般的外伤,鳞、鳍破损等,是小毛病,不会影响到繁殖的质量。养种鱼要注意四个问题:一是要加大换水量,以少次多换为好;可以掌握在三天一次,每次30%;其目的在于刺激鱼的代谢功能。二是要注意防病防虫,燕鱼一旦发病,是不好治的,主要是不赖药,要以预防为主。燕鱼最大的病害是体内虫和肠炎,新鱼稳定后,先要喂药饵驱虫,第一次在稳定7-10天时,以后两个月一次,每次连喂三天,每天喂一次就可以了。预防肠炎也用药饵,每月一次,每次二天。小剂量用药是不会影响繁殖的。(药饵我用的是天津产的“珍宝”2号、3号三是要坚持饵料的多样性,避免单一,防止种鱼营养不良。四是要避免高温饲养,一般控制在25-26℃为好,高温对繁殖和种鱼的寿命都不利。种鱼养好了,配对是很容易的,就让他们自然配对好了,不必太苛求。自然配对一般发生在6、7个月大时,但配对不等于就要繁殖,还要再等1、2个月才行。如果你想定向繁殖,也可以两公一母混养,强制配对,时间掌握在6、7个月大,发现有自然配对现象时,成功率在90%左右。二、燕鱼的繁殖周期关于燕鱼的成熟期的问题,鱼友也很关心。有些资料说是6个月,也有说是10个月的;我掌握的时间是8-10个月,因不同品种和个体而有所差异,但多数是8个月;而公母间的差异不大,基本上是同步的,只有极少的公鱼会晚一些,有母鱼产两次卵,而公鱼不能受精的,这种情况很少遇到(我只遇到一次),如果遇到了也不用急,等他几天就是了,没有什么损失,因为燕鱼是极高产的品种. 关于燕鱼的繁殖周期和产卵量的问题。在人工饲养条件下,燕鱼的繁殖周期并不明显,他们一旦进入成熟期后,就会不停的产卵,并无季节性可言;在一年中会有二、三次间歇,每次30-40天不等,应该是种鱼的恢复期。在产卵期,两次产卵之间的间隔也不固定,少的只有5天,多的有15天,但多数是8-10天,一个产卵周期能产15-20窝左右,每窝的产卵量大约是400-600粒。从开始产卵到第三、四窝时,产卵量达到最高峰,大约从第四个产卵周期开始产卵量逐渐减少,但这种减少并不十分明显。我最近记录了我的一对墨燕的产卵情况,他们是今年8月份开始产卵的,我从10月13日产卵开始记录,他们于10月13日下午、22日下午、27日下午、11月3日下午分别产下四窝卵,时间间隔分别为:9天、5天、7天,每窝产卵量在600粒左右;每窝卵都是48小时孵化,第7天下板。繁殖环境:繁殖缸50*35*40,上过滤,水温27℃,PH7.0,硬度10.0;用10*25厘米的绿色塑料产板,放置角度10度,离底,产板的上边在水面下3厘米左右;卵在孵化桶中人工孵化;喂颗粒饲料和自制汉堡;繁殖缸三天换水30%,直接用的自来水。幼鱼喂丰年虾幼虫,成活率在95%以上。三、燕鱼的繁殖o 繁殖的环境前面说了,不再重复,我不能肯定是不是最佳的环境,但肯定是有效的、成功的。在这里要强调的是——水,平时饲养用什么水,繁殖就用什么水,水温比平时高1℃就可以了,并按正常换水。我平时养鱼用的是自来水,所以繁殖也直接用自来水;换水时不加温,让繁殖缸的温度产生波动,这样对种鱼有很好的刺激作用。但要特别注意加水的速度,一定要慢,应控制在排水速度的一半左右,不能直接冲到鱼,可以加到上滤盒中缓冲一下。卵床用产板最好,我用过盆、罐、筒等,都可以,但燕鱼还是更喜欢板,对板的材料并不苛求,只要平整光滑就行;最好是长20-25厘米、宽8-10厘米的绿色塑料板,10-20度倾斜放置(模仿水草叶片的自然角度),下面离开缸底,产板的上边在水面下3厘米左右,燕鱼的卵一般产在水面下5-20厘米范围内。据我的观察,燕鱼产卵的时间一般都是在下午4-5点钟。四、鱼卵的孵化产卵结束后,如何孵化是个小问题,你可以不管他,
鱼类人工繁殖 百科名片 鱼类人工繁殖 人工繁殖方法按亲鱼来源于天然水域或人工培育,可分为半人工繁殖和全人工繁殖。前者受捕捞水域和季节的限制性大,生产不稳定。后者从亲鱼培育至鱼苗孵出都在人工控制下进行,可有计划地大量生产鱼苗。促使亲鱼成熟、产卵的方法,一般可分为生态法、生理法和生态生理结合法。 目录 鱼类人工繁殖 原理 人工繁殖方法 环节 编辑本段鱼类人工繁殖 fish,artificial propagation of 根据鱼类自然繁殖习性,在人为条件下控制鱼类发育、成熟、产卵和孵化的技术措施。编辑本段原理 鱼类自然繁殖是在水温、水流、溶氧、光照、水位变化,以及性引诱和卵的附着物等外界条件制约下进行的。当这些生态条件综合作用下刺激成熟亲鱼的感觉器官时,鱼即产生冲动,并通过神经纤维传入中枢神经,刺激下丘脑促使释放激素,使脑垂体间叶分泌促性腺激素,使卵细胞发生显著变化。在卵母细胞成熟变化过程中,滤泡膜破裂并进行排卵和产卵;雄鱼的精液量显著增加,并出现性行为。由于池塘内缺乏相应的鱼类繁殖生态条件,不能适度地刺激亲鱼的下丘脑释放激素,从而不能促使亲鱼的垂体分泌一定浓度的性腺激素,使亲鱼自然产卵。因此,人工繁殖的要领就在于将催情剂(如鱼的脑下垂体抽提液、人绒毛膜促性腺激素或促黄体生成素释放激素类似物)注入鱼体,达到诱导亲鱼发情、产卵或排精的目的。 编辑本段人工繁殖方法 生态法是在鱼类繁殖的适温季节里,选择成熟的亲鱼进行雌雄配对,满足其产卵的生态条件,使亲鱼自行繁殖或进行人工采卵和受精。此法适用于鲤、鲫、非鲫和虹鳟等。生理法是在繁殖季节对某些无法满足其成熟产卵生态条件、而性腺发育良好的亲鱼注射催情剂,促其性成熟、产卵和排精,适用于草鱼、青鱼、鲢、鳙等养殖鱼类、印度鲤科鱼类、闪光鲟和鮻等。生理生态法是将上述两法结合运用,既注射催情剂又提供合适的生态条件。 编辑本段环节 中国鲤科养殖鱼类的繁殖一般采用人工繁殖生理生态法,整个过程分亲鱼培育、催情、授精和孵化4个环节。 ①亲鱼培育。是将达到性成熟年龄的亲鱼培育至性腺发育成熟的过程。鱼类成熟年龄因种类和所处纬度而异;雌鱼比雄鱼早熟1龄。亲鱼达到性成熟年龄后,性腺发育要求提供适口而营养丰富的饵料。在进行人工繁殖前约1个月,应适当减少投饵和施肥量,并每日冲注新水4~6次,促其性腺进一步发育。性腺发育良好的雌鱼在外形上腹部膨大、下腹松软、
中文名称:鱼类繁殖 拼音:yuleifanzhi 外文名称:reproductiveof fish 正文:鱼类重要的生命活动,通过这一活动,鱼类才得以保存和繁衍。由于各种鱼类进化进程的差异和对环境适应形式的不同,其繁殖特点也不同。 性分化受精卵发育至形成各胚层时即出现原始性细胞。随着性细胞的增殖与分化,逐渐发育形成生殖腺。鱼类性分化是一个复杂的生物学过程,多数鱼类经过性分化之后呈雌雄异体,但不少种类却形成雌雄同体。 性成熟鱼类在胚胎发育过程中,原始性细胞集中于腹腔,经性分化发育成熟达生殖状态,称为初次性成熟。不同种类初次性成熟时间不同,同一种类不同种群、同一种群雌雄之间也有不同。同一种鱼初次性成熟时间一般与体长有关,生理年龄并不经常反映性成熟的前后顺序。生长越快,达到性成熟时间越短,同一世代体长生长的差异,造成一个世代初次性成熟年龄的不同。 生殖周期与产卵类型不同种类生殖周期的长短不同,雌雄鱼也有差别,同一个体高龄阶段生殖周期往往会延长。多数鱼类生殖周期1年左右,但如尼罗罗非鱼(Oreochromis niloticus)仅30-60天,一年繁殖多次,鲟科(Acipenseridae)鱼类则达2年或更长。鱼类的产卵类型中,按世代分有一生产卵1次、一生多次产卵;按产卵季节分为一个产卵季节一次、一季多次分批产卵。同一种类分布区域不同,产卵类型也可能会改变。 生殖方式和生殖过程多数鱼类行体外受精,少数行体内受精。体外受精鱼类雌雄鱼各自将成熟性细胞排出体外,在水中受精,发育和孵化。体内受精鱼类成熟卵在母体内受精,为把成熟镜子输送雌鱼体内,雄鱼一般具有特化的交接器,如软骨鱼类雄鱼腹鳍内侧鳍条特化呈生殖足。体内受精鱼类胚胎发育有卵生,卵胎生和胎生三种类型:(1)卵生,卵在输卵管内受精,受精卵由卵壳包裹,由卵黄供应养分在水中发育孵化。(2)卵胎生,成熟卵在母体生殖道内受精,由卵黄和母体共同供给养分,产出体外时已长成幼鱼。(3)胎生,成熟卵在母体生殖道内受精发育,由母体供应养分,幼鱼孵化成形后排出体外。 生殖过程中,生殖鱼群依体长大小,组群的现象有明显差异。个体大小不同,在产卵场分布的位置也有不同。亲鱼体长--年龄不同,性细胞质量有差异。一般以中龄大型鱼性细胞的质量最高,低龄鱼或高龄鱼亲鱼较差,其受精率、孵化率和后代成活率均不高。因此,生殖初—中期后代质量和后代成活高于后期产出的后代。每一生殖季节多次分批产卵的鱼类,每批卵子质量也有差别,尤其以第一批卵子质量为高。鱼类一般以高生殖力补偿早期发育阶段的高死亡率。生殖力低的鱼类,除胎生和卵胎生种类后代在母体度过早期发育阶段外,多数种类有护幼习性。如营巢筑穴、口孵哺育,以提高成活率。 产卵期和产卵场鱼类在一年中某一特定季节生殖,是保障其幼体有最良好生长发育条件的一种适应性。幼体生长发育过程一般与水域中饵料生物繁殖状况相一致,也是其幼体发育所需环境条件(尤其是水温、光照周期)和避开敌害的最佳时期。根据生殖期间水温和光照周期变动趋势,鱼类生殖腺存在两种类型:水温上升期和水温下降期。除热带水域外,春季到夏季,水温上升,一天内光照时间延长。秋季到冬季,水温下降,光照时间缩短。在同一生殖期类型内,生殖期
第27卷第8期2008年8月 水产科学 F IS HER IES SC I EN CE V o.l 27No .8 Aug .2008 鱼类原生殖细胞的研究进展 苏 敏,林丹军,尤永隆,唐良华 (福建师范大学生命科学学院,福建 福州 350108)) 摘 要关键词 :原生殖细胞;鱼类;进展中图分类号:Q 959.4 文献标识码:C 文章编号:1003-1111(2008)08-0427-03 收稿日期:2008-01-10; 修回日期:2008-02-29. 基金项目:福建省自然科学基金资助项目(X0650044,2006J 0334,B0710023).作者简介:苏敏(1979-),女,讲师,博士研究生,研究方向:发育生物学;E-m ai:l s um in @fj https://www.doczj.com/doc/2d6975279.html, .cn .通讯作者:唐良华(1974-), 男,副教授,研究方向:生物技术;E-m ai:l b i otl h @163.co m. 除单细胞动物外,动物体由两部分细胞组成:体细胞和 生殖细胞,二者均由同一个受精卵发育而成。现在学术界通常认为:对于大多数动物来说,生殖细胞并非是在胚胎发育的后期才在生殖腺中产生的,而是在胚胎发育的一开始就与体细胞分开了,那时它被称为原生殖细胞(PGCs)。随着胚胎发育的进行,PGCs 通过不同的途径逐渐迁入正在发育的生殖腺中,随后与其共同分化成为精巢或卵巢[1]。 PGC s 研究的取材对象主要集中于两栖类、爬行类、鸟类、哺乳类等,对于鱼类这方面的研究则相对较少,所得意见也不太统一[2]。研究主要围绕PG Cs 的起源、迁移和分化的路线进行;此外,PGC s 的形态特征、数目、迁移的机制、增殖的机制以及对PGCs 产生影响的一些生长因子或外源理化因子等均属于其研究的范围。在国内,20世纪80年代前,对鱼类的PG Cs 的研究还是一个空白;80年代以来,在这方面的研究开始有所报道,如刘少军、张杨宗等[3-4]对革胡子鲶(C lar i as l azera )、草鱼(C tenophary ngodon i dellus )的PGC s 的研究,开始揭示了鱼类的PGC s 的一些超微结构;但是,迄今为止,国内鱼类PGCs 的研究仍鲜见报道,并且研究手段迟滞不前。近年来,斑马鱼(D anio rerio )生殖细胞中特有的V asa 基因在国外克隆成功[5-7],开创了以分子生物学技术研究鱼类PGCs 的新纪元。PG Cs 的发生是性腺发育和成熟的基础,对鱼类的PGCs 的研究无论在理论上还是在生产中均具有重要的意义。 1 鱼类PGCs 均的特征 要了解PG Cs 的数目、起源、迁移和分化,首先要掌握PGC s 的特征。迄今为止,主要还是通过结合细胞的显微结构和亚显微特征来进行辨认。 N edelea 等[8]最早在鲤(Cyp rinus carp io )PGC s 胞质中发现了电子致密体(E l ectro dense bod i es )。Satoh [9]以青鳉(O riz i as l ati p es )为材料对电子致密体作了较为详细的描述:电子致密体直径为1.0 m,无膜包被,由非常纤细的原纤维交织成网,常与线粒体群紧密相连并直接接触线粒体外膜。H og an [10]在青鳉预定内胚层细胞中也发现了类似的细胞质标志性结构。此外,H a m aguchi [11]在日本阔尾鳉(https://www.doczj.com/doc/2d6975279.html,ti p es ),B illard [12]在网纹食纹鱼(P oecilla reticulata ),刘少军[3]在革胡子鲶PG Cs 的超微结构中也发现这种与线粒体相联系的电子致密体。鱼类PGCs 中的这种结构与两栖类爪蟾(X enopus laevis )中PGCs 的生殖质在结构上十分相似,而两栖类爪蟾PGC s 的生殖质中含有RNA,可能与蛋白质的合成有关。Hogan [10]认为鱼类PGCs 的超微结构除了有与线粒体相联系的电子致密物这一特征外,还有另一个特征是与细胞核膜相联系的弯曲的内质网结构[10]。 G amo [13]证实,鱼类局部卵裂胚胎中PGC s 中含有卵黄块,其含量少于完全卵裂的胚胎。这表明,真口类(真骨鱼类)(T eleosto m i )PGC s 的结构与营养资源或表胚层(Per-i blast)有关。M aschko w zeff [14]也在鲟(A ci p enser st urio )早期胚胎的PGC s 细胞质中也发现有卵黄块,并且随着胚胎发育,其卵黄块逐渐减少,直致消失。刘少军[3]在革胡子鲶中有同样发现,由于此时的PGCs 靠近卵黄囊,他认为这可能与PGCs 在迁移过程中需要消耗卵黄作为营养物质有关。 一般认为鱼类PGC s 与普通细胞相比,具有较大的体积和核质比例较低的特点。PGCs 比普通细胞体积大的主要原因是它们很少发生有丝分裂。但是在发育过程中,有卵黄块的PGC s 会因其细胞质内的卵黄物质被吸收而使体积有所减小,PG Cs 的核质比也会随着细胞质的减小和卵黄物质的被吸收而增大。不同鱼类PG Cs 的大小与它们卵子的大小之间无明显的相关,但是一般认为较大的PGCs 存在于较原始的鱼类中,如:大马哈鱼(O ncorhynchus keta )、鲟、弓 鳍鱼(Am i a clava )的PGC s 的体积均较大[15] 。 鱼类PGC s 具有以下共同的特征:(1)鱼类PG Cs 周围的细胞大,呈圆形、卵圆形或梨形,细胞界线明显,细胞直径因其形状、发育期和种属的不同而异;(2)鱼类PG Cs 的细胞核很大且明亮,呈圆形、卵圆形或分叶状,核膜清晰,核常位于细胞一侧。核仁明显,1~2个;(3)鱼类PGC s 的超微结构中,区别于其他细胞,具有与线粒体相联系的电子致密体;(4)鱼类PGCs 在迁移期可见叶状伪足和丝状伪足。 2 鱼类PGC s 的起源、迁移和分化 2.1 鱼类PG Cs 的起源 根据已有的资料分析,对于鱼类PGCs 的起源,虽然意见不一,但是主要有两种观点:一种认为起源于中胚层,另一种认为起源于内胚层。 O kke l berg [16]研究普氏七鳃鳗(E ntosphennus w ilderi )PGCs 起源时发现,PGC s 最早来自早期尾芽期躯干尾区的侧板中胚层,稍后汇集在吴尔夫管(W o lffi an duct)附近;N ede lea 等[8]发现,鲤(Cyp rinus )属PGCs 始见于卵黄外包完成不久前的胚盾中胚层;高书堂等[17]对泥鳅(M isgurnus an -gu illicaudatus )的观察结果则是,泥鳅PGC s 最先出现在原肠晚期的预定中胚层区域。这些试验结果均提示鱼类PG Cs 起源于中胚层;此外,中胚层起源说的另一个强有力的试验依据是:Oppenhe i m er [18]在底鳉属(Fundulus heteroclit us )原肠中期,将取自后1/3胚盾的随机重组片断植入宿主胚外区后,结果可在无任何内胚层结构中形成带有PGCs 的生殖腺。 然而,持内胚层起源说的学者也不在少数。A ll en [19]在雀鳝属(L e p isos teus )发现,其PGCs 位于单层肠内胚层的腹部和侧部;M aschkowzeff [14]也在鲟属的肠内胚层中发现PGCs ;刘少军、林光华等[3,20]在研究革胡子鲶后得出的结论是,革胡子鲶PGCs 起源于原肠期胚胎内胚层。 还有一点要提及的是,一些研究发现,在某些鱼类的PGCs 似乎与多核表胚层(Syncytial pe ri b l ast)有关。R e i n -
《鱼类组织胚胎学》试卷(A)参考答 案 班级姓名序号分数 一、名词解释(每题 4 分,共 20 分): 1、尼氏体— 2、肌纤维— 3、肠绒毛— 4、肾小球— 5、合子— 二、填空题(每空0、5分,共 15 分):
1、衬贴在心血管及淋巴管内表面的单层扁平上皮称,分布在浆膜表面的单层扁平上皮称为; 2、结缔组织 有、、等三种纤维成分; 3、肌组织可分 为、和三种类型; 4、鱼的辅助呼吸主要有_ _ _,_ _,_ _, ; 5、消化管壁分四层, 即_ , _,_ ,_ ; 6、胃腺中分泌胃蛋白酶原的细胞是,分泌盐酸的细胞是; 7、胰腺是既有外分泌腺,又有内分泌腺的一种混合腺体,其中主要部分呈管泡状结构,称胰腺腺泡,为,另有一些呈岛屿分布,称胰岛,为;
8、肾单位由_ _、__ _组成,肾小体有两个极,即 _ _、 _ ,前者 是_出入处, 后者与_ 相连; 9、内分泌腺没有__ _,其分泌物(激素)由腺上皮分泌后直接进入_ _; 10、生殖细胞在成熟期内要经过两次成熟分裂,其中第一次成熟分裂为,第二次成熟分裂。 三、判断题(正确的划“√”,错误的划“×”,每题2分,共20分): 1、肠粘膜柱状上皮间有一种杯状细胞,又称粘液细胞,属于一种单细胞腺体;() 2、由内外分泌腺所分泌的分泌物称为激素;() 3、哺乳动物成熟红细胞无细胞核,鱼类红细胞也无细胞核;() 4、骨骼肌与心肌都有横纹,都属于随意肌;() 5、血管的管壁都是由内膜、中膜和外膜三层结构组成;() 6、微绒毛是指肠粘膜上皮和粘膜固有层向肠腔内折起形成的指状突起;()
7、腮小片是腮丝表面形成的微细的片状突起,为鱼类气体交换 的基本单位;() 8、肾小体是由肾小球和肾小管组成;() 9、精子的两次成熟分裂都是在精巢的精小囊内进行;() 10、血浆是由血清和纤维蛋白原组成;() 四、选择题(每题1分,共10分): 1.假复层柱状纤毛上皮分布于:() A.气管;B.食管;C.膀胱; D.肠 管。 2.血液中数量最多的白细胞是:() A. 嗜中性白细胞;B.淋巴细胞;C.单核细胞;D.嗜酸性粒细胞。 3.具有吞噬功能的细胞是:() A.成纤维细胞;B.巨噬细胞;C.浆细胞;D.肥大细 胞。 4.合成和分泌免疫球蛋白的细胞是:() A.成纤维细胞;B.浆细胞;C.巨噬细胞;D. 肥大细胞。 5.肾脏具有回收作用的部位主要是:() A.近曲小管;B.远曲小管;C.肾小囊;D.集合管。
第五章鱼类的繁殖 繁殖是鱼类生命过程中的一个重要环节,是维持种族绵延永盛不可缺少的生命活动。掌握鱼类的繁殖习性和性腺的发育规律,对于研究鱼类的人工繁殖、选种与育种、移植驯化及鱼类资源的合理开发利用具有十分重要的意义。 第一节鱼类的性腺发育与性成熟 所谓性腺是指鱼体内产生殖细胞的组织。绝大多数鱼类为雌雄异体。雌鱼的性腺为卵巢,雄鱼的性腺为精巢。性腺由卵巢系膜或精巢系膜悬系于腹腔背壁,位于消化道背侧、鳔腹面两侧,呈长囊状或圆柱形,一般成对,左右对称,成熟的生殖细胞通过生殖导管(输卵管或输精管)输送到体外。 一、性腺发育 (一)卵巢的结构和发育 1.卵巢的结构 卵巢是雌性鱼类的生殖腺,是产生卵子的器官。鱼类的卵巢一般成对,多数左右明显分开。有的种类则完全合并,如河鲈;有的在后部合并,如梭鲈;有的在中部合并,如条鳅。也有的左右不对称,如香鱼、池沼公鱼;有的只有一个,如黄鳝的左侧卵巢发达,右侧退化。在未成熟时呈半透明的条状,成熟时则呈长囊状。颜色多为黄色,也有的种类因卵的颜色不同而呈现其他色泽,如鲶鱼成熟的卵巢呈绿色,大麻哈鱼成熟的卵巢呈橘红色。根据卵巢外方腹膜的有无及输卵管与卵巢是否相通等特点,可以将鱼类的卵巢分为游离卵巢和封闭卵巢两种类型。 (1)游离卵巢又称为裸卵巢,即卵巢不为腹膜形成的卵巢囊所包围。这种类型的卵巢一般不与输卵管直接相连,成熟卵先排入腹腔中,再经过输卵管腹腔口进人输卵管。一般认为游离卵巢代表原始类型的构造,如圆口类、板鳃类、全头类、硬鳞类等的卵巢。 (2)封闭卵巢又称为被卵巢,卵巢被腹膜所形成的卵巢囊所包围,卵巢囊上有环肌和纵肌,其收缩可排卵。成熟的卵子一般不排到体腔中,而是直接落如卵巢中的卵巢腔内,卵巢囊后部变狭成为输卵管。这是高级类型的卵巢构造,真骨鱼类的卵巢属此类型。封闭卵巢外层腹膜下有一薄层由结缔组织构成的白膜,它向卵巢腔内部伸出许多由生殖上皮、结缔组织和微血管组成的板层状结构,它们是产生卵子的地方,称为产卵板。 图5-1 几种鱼类的雌性生殖器官 2.卵巢的发育分期 多数硬骨鱼类的卵巢发育过程,依据性腺的体积、颜色、血管分布、性细胞成熟与否等标准,通过目测,一般分为6个时期。 I期卵巢:性腺未成熟,紧贴于鳔下两侧的体腔膜上,呈透明的细线状,看不见卵粒,肉眼不能分辨雌雄。这是鱼类第一次性成熟过程中所特有的阶段,一般当年家鱼的性腺处于此期。
中文名:鱼类性腺成熟度 拼音:yuleixingxianchengshudu 外文名称:maturity of fish gonad 正文:鱼类性腺成熟度是根据鱼类性腺外表性状和性细胞发育程度所划分的性腺发育等级。性腺发育成熟过程是鱼类把摄食食物所获得的物质和能量资源分配到性腺的过程,该过程占整个繁殖过程资源的大部分。鱼类性腺成熟过程还包括亲鱼的第二性征和繁殖行为所消耗的能量。研究鱼类的性腺发育以及影响和控制性腺发育的因子,对于鱼类繁殖生态学理论研究,以及掌握人工繁殖时间,掌握鱼汛和中心渔场的变动具有重要意义。 判断鱼类性腺成熟度是水产资源调查研究的最常规项目之一,其划分一般有四种方法,分别为目测等级法、组织学划分法、卵径分布法和性成熟系数法。(1)目测等级法主要依据性腺的外形、色泽、血管分布、卵与精液的情况等特征进行判断。该方法的将性腺成熟度划分为六期,具体标准如下:I期:性腺尚未发育的个体。性腺不发达,紧附于体壁内侧,呈细线或细带状,肉眼不能辨别雌雄。 II期:性腺开始发育或产卵后重新发育的个体。 III期:性腺正在成熟的个体。性腺已较发达,卵巢体积较大,占整个腹腔的1/3-1/2。IV期:性腺即将成熟的个体,卵巢已有很大的发展,占腹腔的2/3左右,分支血管可以明显看出,卵粒显著,呈圆形。 V期:性腺完全成熟,即将或正在产卵个体。卵巢饱满,充满体腔。 VI期:产卵排精后的个体。性腺萎缩、松弛、充血、,呈暗红色,体积显著缩小,占体腔的一小部分。 (2)组织学划分法鱼类卵子的发生要经过增殖、生长和成熟几个时期。随季节变化或性周期运转,在卵巢发育过程中,可观察到不同时期(相)的生殖细胞。具体划分过程如下: 1)第1时相这是处于卵原细胞(oogonium)阶段或由卵原细胞向初级卵母细胞(oocyte of the first order)过渡的细胞。主要见于未成热的卵巢。 2)第2时相即处在小生长期的初级卵原细胞。其体积较第一时相时大,按其形态变化可分成早、中、晚三个阶段。 3)第3时相即处在大生长期早期的初级卵母细胞。细胞大多呈圆球形,排列松散。 4)第4时相即处在大生长期晚期的初级卵母细胞。由于卵黄物质的不断积聚,卵母细胞的体积迅速增大,以其形态变化及卵径大小,可分早、中、晚三个阶段。 5)第5时相,即已达成熟阶段的卵细胞。 (3)卵径分布法从卵细胞发育趋势而言,随着性腺发育,卵径亦趋增大。因此,如能逐月测定卵巢内卵径大小的频率分布变化,即可判断鱼类的产卵类型、产卵期以及随鱼休大小变化的规律。如石岛渔场高眼蝶产卵群体的卵径分布表明,该鱼在4、5月产卵,属于一次产卵类型,且随年龄增长,卵径分布趋势亦随之上升(图1—1)。